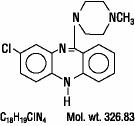
Clozapine
What is Clozaril (Clozapine)?
Living with schizophrenia can be a deeply challenging journey, both for patients and their loved ones. While many antipsychotic medications can help manage symptoms, some people do not respond adequately to standard treatments. For those individuals, Clozapine offers a proven, life-changing option.
Clozapine is an atypical antipsychotic medication primarily used to treat schizophrenia that hasn’t improved with other drugs. It is also sometimes prescribed for reducing suicidal thoughts in people with schizophrenia or schizoaffective disorder. Though it requires close medical supervision, Clozapine has shown exceptional effectiveness in helping patients regain stability, clarity, and a better quality of life.
What does Clozapine do?
Clozapine helps control symptoms of schizophrenia, such as hallucinations, delusions, disorganized thinking, and emotional withdrawal. For many patients who haven’t responded to other medications, it can offer significant improvement in both symptoms and day-to-day functioning.
It is especially effective in:
- Treatment-resistant schizophrenia, when two or more antipsychotic drugs have failed to provide relief.
- Reducing suicidal behavior in people with schizophrenia or schizoaffective disorder.
Clinical studies have consistently shown Clozapine’s superior efficacy in improving both positive symptoms (like hallucinations or paranoia) and negative symptoms (such as apathy or lack of emotion) compared to other antipsychotic drugs (Kane et al., 1988). Many patients experience not just symptom control but also improved engagement in daily life and reduced hospitalization rates.
Because of its unique benefits, Clozapine is often regarded as a “last-line but best-performing” antipsychotic when others fail.
How does Clozapine work?
Clozapine works by balancing certain brain chemicals called neurotransmitters, especially dopamine and serotonin. In schizophrenia, overactivity of dopamine in certain brain regions is thought to contribute to hallucinations and delusions. Clozapine helps modulate dopamine activity rather than completely blocking it, which makes it more effective and better tolerated than older antipsychotics.
It also affects serotonin receptors, which contributes to mood stabilization and reduced anxiety. This dual action is clinically important because it helps reduce psychotic symptoms with less stiffness or tremor than older drugs, improve mood, thinking, and motivation, and lower the risk of relapse for better long-term stability.
Clozapine’s effectiveness stems from this broad yet balanced mechanism, targeting multiple pathways involved in schizophrenia while minimizing certain neurological side effects seen with other treatments.
Clozapine side effects
Clozapine is a powerful and effective medication, but it does carry potential side effects that require careful medical monitoring.
Common side effects include:
- Drowsiness or fatigue
- Increased saliva production
- Weight gain
- Constipation
- Dizziness when standing up quickly
Less common side effects:
- Fast heartbeat
- Mild fever
- Muscle stiffness
- Tremors
Serious side effects (require immediate medical attention):
- Agranulocytosis, a rare but serious drop in white blood cells that can increase infection risk. This is why regular blood testing is mandatory.
- Seizures, particularly at higher doses.
- Myocarditis (heart inflammation) or cardiomyopathy.
- Severe constipation that may lead to bowel obstruction.
Because of these risks, Clozapine is prescribed under a special monitoring program known as the Clozapine Risk Evaluation and Mitigation Strategy (REMS) in the United States.
You should not take Clozapine if you have:
- A history of low white blood cell counts or bone marrow disorders.
- Uncontrolled epilepsy.
- Severe heart or intestinal problems.
Seek medical care immediately if you develop symptoms such as sore throat, fever, mouth ulcers, chest pain, or persistent constipation. These may indicate serious side effects that need prompt evaluation.
Clozapine dosage
Clozapine is available as an oral tablet, orally disintegrating tablet, or oral suspension. It is typically started at a low dose and gradually increased to minimize side effects such as dizziness or sedation.
Because of its potential to affect white blood cell counts, regular blood monitoring is mandatory. Your doctor will order frequent blood tests typically:
- Weekly for the first 6 months
- Every 2 weeks for the next 6 months
- Monthly thereafter, as long as blood counts remain stable
In addition to blood tests, your doctor may check:
- Heart function (ECG or echocardiogram) to detect inflammation
- Weight, cholesterol, and blood sugar levels, as Clozapine can affect metabolism
For older adults, people with diabetes, or those with heart disease, extra care is taken in dosing and monitoring to ensure safety.
Taking Clozapine exactly as prescribed and never stopping it abruptly is crucial. Stopping suddenly may cause withdrawal symptoms or recurrence of psychosis.
Does Clozapine have a generic version?
Yes, Clozapine is available as a generic medication, approved by the U.S. Food and Drug Administration (FDA). Generic Clozapine offers the same quality, safety, and effectiveness as brand-name versions but is often more affordable.
Common brand names include Clozaril, Versacloz, and FazaClo. All contain the same active ingredient, clozapine, but differ in form, tablets, liquids, or orally disintegrating tablets.
Patients can safely use the generic version if prescribed by their healthcare provider. Always confirm that your pharmacy dispenses an FDA-approved product to ensure consistent treatment quality.
Conclusion
Clozapine remains one of the most effective medications for treatment-resistant schizophrenia, offering hope to individuals who have not found relief with other antipsychotics. Its ability to control both hallucinations and emotional symptoms can dramatically improve quality of life and long-term outcomes.
Clozapine, while requiring careful monitoring due to potential side effects, often offers benefits outweighing risks when diligently prescribed and followed. Successful treatment hinges on open communication, adherence to tests, and awareness of warning signs.
Clozapine is safe and effective when prescribed and monitored by a qualified healthcare provider. With proper care, it can be a transformative part of recovery, helping patients reclaim stability, confidence, and connection in daily life.
References
- Kane, J. M., et al. (1988). Clozapine for the treatment-resistant schizophrenic: A double-blind comparison with chlorpromazine. Archives of General Psychiatry, 45(9), 789–796.
- U.S. Food and Drug Administration (FDA). (2024). Clozapine REMS Program Overview. https://www.fda.gov/
- Mayo Clinic. (2024). Clozapine (Oral Route) Description and Precautions. https://www.mayoclinic.org/
- MedlinePlus. (2024). Clozapine – Drug Information. https://medlineplus.gov/
- National Institute of Mental Health (NIMH). (2023). Schizophrenia Treatment and Medications. https://www.nimh.nih.gov/
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- Severe Neutropenia
- Orthostatic Hypotension, Bradycardia, and Syncope
- Falls
- Seizures
- Myocarditis, Pericarditis, Cardiomyopathy, and Mitral Valve Incompetence
- Increased Mortality in Elderly Patients with Dementia-Related Psychosis
- Gastrointestinal Hypomotility with Severe Complications
- Eosinophilia
- QT Interval Prolongation
- Metabolic Changes (Hyperglycemia and Diabetes Mellitus, Dyslipidemia, and Weight Gain)
- Neuroleptic Malignant Syndrome
- Hepatotoxicity
- Fever
- Pulmonary Embolism
- Anticholinergic Toxicity
- Interference with Cognitive and Motor Performance
- Tardive Dyskinesia
- Cerebrovascular Adverse Reactions
- Recurrence of Psychosis and Cholinergic Rebound after Abrupt Discontinuation
- Severe Neutropenia:
- About the risk of developing severe neutropenia and infection with CLOZARIL treatment.
- Instruct patients to immediately report to their health care provider any symptom or sign of during CLOZARIL treatment.
- About the importance of having frequent ANC testing.
- Orthostatic Hypotension, Bradycardia, and Syncope: Inform patients and caregivers about the risk of orthostatic hypotension and syncope, especially during the period of initial dose titration. Instruct them to strictly follow the clinician’s instructions for dosage and administration [see Dosage and Administration (2.6)]. Advise patients to consult their clinician immediately if they feel faint, lose consciousness or have signs or symptoms suggestive of bradycardia or arrhythmia [Warnings and Precautions (.
- Seizures: Inform patients and caregivers about the significant risk of seizure during CLOZARIL treatment. Caution them about driving and any other potentially hazardous activity while taking CLOZARIL [see Warnings and Precautions (.
- Gastrointestinal Hypomotility with Severe Complications: Educate patients and caregivers on the risks, prevention and treatment of clozapine-induced constipation, including medications to avoid when possible (e.g., drugs with anticholinergic activity). Encourage appropriate hydration, physical activity, and fiber intake and emphasize that prompt attention and treatment to the development of constipation or other gastrointestinal symptoms is critical in preventing severe complications. Advise patients and caregivers to contact their health care provider if they experience symptoms of constipation (e.g., difficulty passing stools, incomplete passage of stool, decreased bowel movement frequency) or other symptoms associated with gastrointestinal hypomotility (e.g., nausea, abdominal distension or pain, vomiting) [see Warnings and Precautions (.
- QT Interval Prolongation: Advise patients to consult their clinician immediately if they feel faint, lose consciousness or have signs or symptoms suggestive of arrhythmia. Instruct patients to not take CLOZARIL with other drugs that cause QT interval prolongation. Instruct patients to inform their clinicians that they are taking CLOZARIL before any new drug [see Warnings and Precautions (.
- Metabolic Changes (hyperglycemia and diabetes mellitus, dyslipidemia, weight gain): Educate patients and caregivers about the risk of metabolic changes and the need for specific monitoring. The risks include hyperglycemia and diabetes mellitus, dyslipidemia, weight gain, and cardiovascular reactions. Educate patients and caregivers about the symptoms of hyperglycemia (high blood sugar) and diabetes mellitus (e.g., polydipsia, polyuria, polyphagia, and weakness). Monitor all patients for these symptoms. Patients who are diagnosed with diabetes or have risk factors for diabetes (obesity, family history of diabetes) should have their fasting blood glucose monitored before beginning treatment and periodically during treatment. Patients who develop symptoms of hyperglycemia should have assessments of fasting glucose. Clinical monitoring of weight is recommended [see Warnings and Precautions (.
- Interference with Cognitive and Motor Performance: Because CLOZARIL may have the potential to impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that CLOZARIL therapy does not affect them adversely [see Warnings and Precautions (.
- Missed Doses and Re-initiating Treatment: Inform patients and caregivers that if the patient misses taking CLOZARIL for 1 day or more, they should not restart their medication at the same dosage but should contact their physician for dosing instructions [see Dosage and Administration (.
- Pregnancy: Advise pregnant women to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with CLOZARIL. Advise patients that CLOZARIL may cause extrapyramidal and/or withdrawal symptoms (agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder) in a neonate. Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to CLOZARIL during pregnancy [see Use in Specific Populations (.
- Lactation: Advise breastfeeding women using CLOZARIL to monitor infants for excess sedation and to seek medical care if they notice this sign. Inform breastfeeding women using CLOZARIL that their healthcare provider will monitor infants for neutropenia[see Use in Specific Populations (
- Concomitant Medication: Advise patients to inform their healthcare provider if they are taking, or plan to take, any prescription or over-the-counter drugs; there is a potential for significant drug-drug interactions [see Dosage and Administration (.
(clozapine)
tablets, for oral use
- Severe neutropenia (low white blood cell (WBC) counts) that can lead to serious infections and death.
- Your healthcare provider will do WBC blood tests before starting treatment with CLOZARIL and weekly for the first 6 months. After your first 6 months of treatment, your healthcare provider will determine how frequent you will have blood tests. If you have symptoms of severe neutropenia or an infection, your healthcare provider will do more frequent WBC blood test(s) to check if CLOZARIL is causing your symptoms and may send you to see a blood specialist (hematologist). Tell your health care provider right away if you have any of the following symptoms or signs of neutropenia or infection:
- Orthostatic hypotension (decreased blood pressure), bradycardia (slow heart rate), or syncope (fainting) that can lead to death. You may feel lightheaded or faint when you rise too quickly from a sitting or lying position. Tell your healthcare provider right away if you feel dizzy or pass out.
- Seizures. See “What should I avoid while taking CLOZARIL?”
- Myocarditis (heart muscle inflammation), pericarditis (inflammation of outer layer of the heart) and cardiomyopathy (heart muscle weakness) that can lead to death. Symptoms of myocarditis, pericarditis, and cardiomyopathy include:
- Increased risk of death in elderly people with dementia-related psychosis. Medicines like CLOZARIL can increase the risk of death in elderly people who have lost touch with reality (psychosis) due to confusion and dementia. CLOZARIL is not for treatment of elderly people with dementia-related psychosis.
- Who are severely ill with schizophrenia not helped by other schizophrenia medicines
- With schizophrenia or schizoaffective disorder who have been suicidal and may be at risk of suicidal behavior again
- are allergic to clozapine or any of the ingredients in CLOZARIL. See the end of this Medication Guide for a complete list of ingredients in CLOZARIL.
- have or have had heart problems or a family history of heart problems including heart attack, heart failure, abnormal heart rhythm or long QT syndrome, or stroke
- have or have had low or high blood pressure
- have or have had kidney or liver problems
- have or have had seizures (convulsions)
- have or have had stomach or intestinal problems including constipation, slow emptying of your stomach, or diarrhea
- have or have had low levels of potassium or magnesium in your blood
- have or have had diabetes or high blood sugar in you or your family
- have or have had high levels of total cholesterol, “bad” cholesterol (LDL-C), or triglycerides, or low levels of “good” cholesterol (HDL-C)
- have increased pressure in your eyes (glaucoma), an enlarged prostate, or problems passing urine
- have or have had uncontrolled movements of your tongue, face, mouth, or jaw (tardive dyskinesia)
- smoke tobacco
- plan to stop smoking tobacco while taking CLOZARIL
- use products containing caffeine
- are pregnant or plan to become pregnant. Talk to your healthcare provider if you become pregnant while taking CLOZARIL.
- are breast feeding or plan to breast feed. CLOZARIL can pass into your breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take CLOZARIL.
- CLOZARIL and other medicines may affect each other causing side effects.
- Your healthcare provider can tell you if it is safe to take CLOZARIL with your other medicines. Do not start or stop any medicines while taking CLOZARIL without talking to your healthcare provider first.
- Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
- Take CLOZARIL exactly as your healthcare provider tells you to take it.
- Take CLOZARIL with or without food.
- If you miss taking CLOZARIL for 1 day or more, call your healthcare provider right away. Do not take 2 doses at the same time unless your healthcare provider tells you to.
- If you take too much (overdose) CLOZARIL, call your healthcare provider or the Poison Help line at 1-800-222-1222 or go to the nearest hospital emergency room right away.
- Symptoms of CLOZARIL overdose can include:
- You should not drink alcohol while taking CLOZARIL because it can increase your chances of getting serious side effects.
- Do not drive, operate machinery, swim, climb, or do dangerous activities until you know how CLOZARIL affects you.
- See
- falls. CLOZARIL may make you sleepy, dizzy, may cause a decrease in your blood pressure when changing positions, and can slow your thinking and motor skills which may lead to falls that can cause fractures or other injuries.
- slow emptying of your stomach and intestines (decreased gastric motility). Severe constipation and bowel problems can happen and can lead to hospitalization, surgery, and death. You may not feel or be aware of constipation symptoms. Your healthcare provider will examine you for possible bowel problems. Tell your healthcare provider if you get any signs and symptoms of decreased gastrointestinal motility during treatment with CLOZARIL, including:
- Staying well hydrated, increasing physical activity, and taking fiber during treatment with CLOZARIL can help prevent constipation and other bowel problems. Your healthcare provider may prescribe medicines to prevent severe problems.
- high count of a certain white blood cell (eosinophilia). CLOZARIL can cause a high count of eosinophils in some people and can be serious. This is a different risk than the risk of CLOZARIL causing an abnormally low white blood cell count (neutropenia). Your health care provider may send you to see an internal medicine specialist (internist) or blood specialist (hematologist). Tell your healthcare provider right away if you have any of these symptoms:
- serious heart rhythm problems (QTc Interval Prolongation) that can cause death. Your healthcare provider will do a physical exam and may obtain blood tests and an electrocardiogram before starting you on treatment with CLOZARIL. Tell your healthcare provider right away if you have any of these symptoms:
- passing out or feeling like you will pass out
- dizziness
- feeling as if your heart is pounding or missing beats
- problems with your metabolism such as:
- high blood sugar (hyperglycemia) or diabetes. Increases in blood sugar can happen in some people who take CLOZARIL. Extremely high blood sugar can lead to coma and death. If you have diabetes or risk factors for diabetes (such as being overweight), your health care provider should check your blood sugar before you start CLOZARIL and during treatment. Tell your healthcare provider if you have any of these symptoms of high blood sugar while taking CLOZARIL:
- increased fat levels (cholesterol and triglycerides) in your blood (dyslipidemia). Your healthcare provider should check the fat levels in your blood before you start and during treatment with CLOZARIL.
- weight gain. You and your healthcare provider should check your weight regularly.
- neuroleptic malignant syndrome (NMS). NMS is a rare but serious condition that can lead to death and must be treated in a hospital. Tell your healthcare provider right away if you become severely ill and have any of these symptoms:
- liver problems. CLOZARIL can cause serious life-threatening liver problems that can lead to death. Tell your healthcare provider right away if you have any of these symptoms:
- fever. Some people may have a fever while they take CLOZARIL. If you have a fever, your healthcare provider will do blood tests to check for neutropenia or an infection. Your healthcare provider may also send you to see a blood specialist (hematologist). Tell your healthcare provider if you have a fever.
- blood clot in your lung (pulmonary embolism) or in the veins of your legs (deep vein thrombosis). Get emergency help right away if you have symptoms of a blood clot including:
- chest pain and shortness of breath
- swelling or pain in your leg, ankle or foot
- warm feeling in the skin of your affected leg
- changes in your skin color such as turning pale or blue
- a problem that includes dry mouth, increased sweating, increased pulse rate, constipation, and urinary retention (anticholinergic toxicity).
- problems thinking clearly and moving your body. See “What should I avoid while taking CLOZARIL?”
- uncontrolled movements of your tongue, face, mouth, or jaw (tardive dyskinesia). Tardive dyskinesia may not go away, even if you stop CLOZARIL. Tardive dyskinesia may also start after you stop taking CLOZARIL.
- stroke (cerebrovascular problems) in elderly people with dementia-related psychosis that can lead to death.
- These are not all the possible side effects of CLOZARIL.
- Your healthcare provider may lower your dose or temporarily or permanently stop treatment with CLOZARIL if you have certain symptoms or if your WBC count is low.
- Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
- You may report side effects to FDA at 1-800-FDA-1088.
- Store CLOZARIL at room temperature between 68°F to 77°F (20°C to 25°C).

