Advise the patient to read the FDA-approved patient labeling (
DRESS/Multi-organ Hypersensitivity
Instruct patients and caregivers that a fever or rash associated with signs of other organ system involvement (e.g., lymphadenopathy, hepatic dysfunction) may be drug-related and should be reported to their healthcare provider immediately. XCOPRI should be discontinued immediately if a serious hypersensitivity reaction is suspected
QT Shortening
Instruct patients to inform their healthcare provider of all of the medications, over-the-counter medications, and herbal supplements that they are taking. Instruct patients to notify their healthcare provider if they have any symptoms of shortening of the QT interval, including prolonged heart palpitations or a loss of consciousness
Suicidal Behavior and Ideation
Counsel patients, their caregivers, and/or families that antiepileptic drugs, including XCOPRI, may increase the risk of suicidal thoughts and behavior, and advise patients to be alert for the emergence or worsening of symptoms of depression; unusual changes in mood or behavior; or suicidal thoughts, behavior, or thoughts about self-harm. Advise patients, their caregivers, and/or families to report behaviors of concern immediately to a healthcare provider
Liver Injury:
Inform patients that liver injury has been reported with XCOPRI. Instruct patients treated with XCOPRI to promptly report any symptoms that may indicate liver injury, including unexplained nausea, vomiting, right upper quadrant abdominal pain, fatigue, anorexia, jaundice, or dark urine. Discuss with patients that a blood test should be obtained before they start therapy if one has not been done within the prior 3 months, and that blood tests will be obtained during treatment if clinically indicated
Neurological Adverse Reactions
Counsel patients that XCOPRI causes somnolence, fatigue, dizziness, and gait disturbance. These adverse reactions, if observed, are more likely to occur early in treatment but can occur at any time. Advise patients not to drive or operate machinery until they have gained sufficient experience on XCOPRI to gauge whether it adversely affects their ability to drive or operate machinery and that other CNS depressants or alcohol may have additive effects
Withdrawal of XCOPRI
Advise patients not to discontinue use of XCOPRI without consulting with their healthcare provider. XCOPRI should normally be gradually withdrawn to reduce the potential for increased seizure frequency and status epilepticus
Contraceptives
Counsel females of reproductive potential that XCOPRI may decrease the efficacy of oral contraceptives and advise them to use additional or alternative non-hormonal birth control
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during XCOPRI therapy. Encourage patients to enroll in the North American Antiepileptic Drug Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy
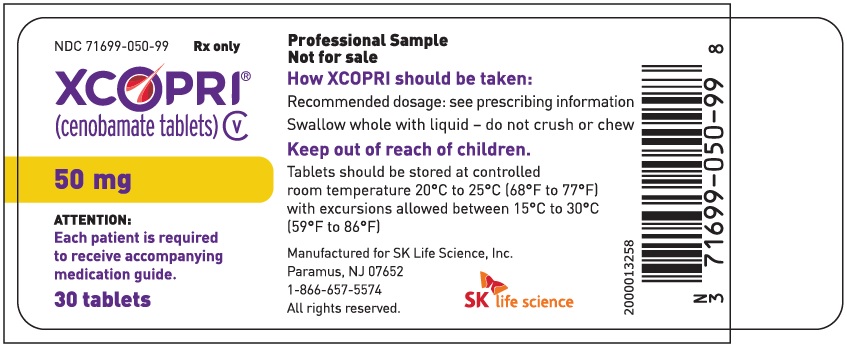
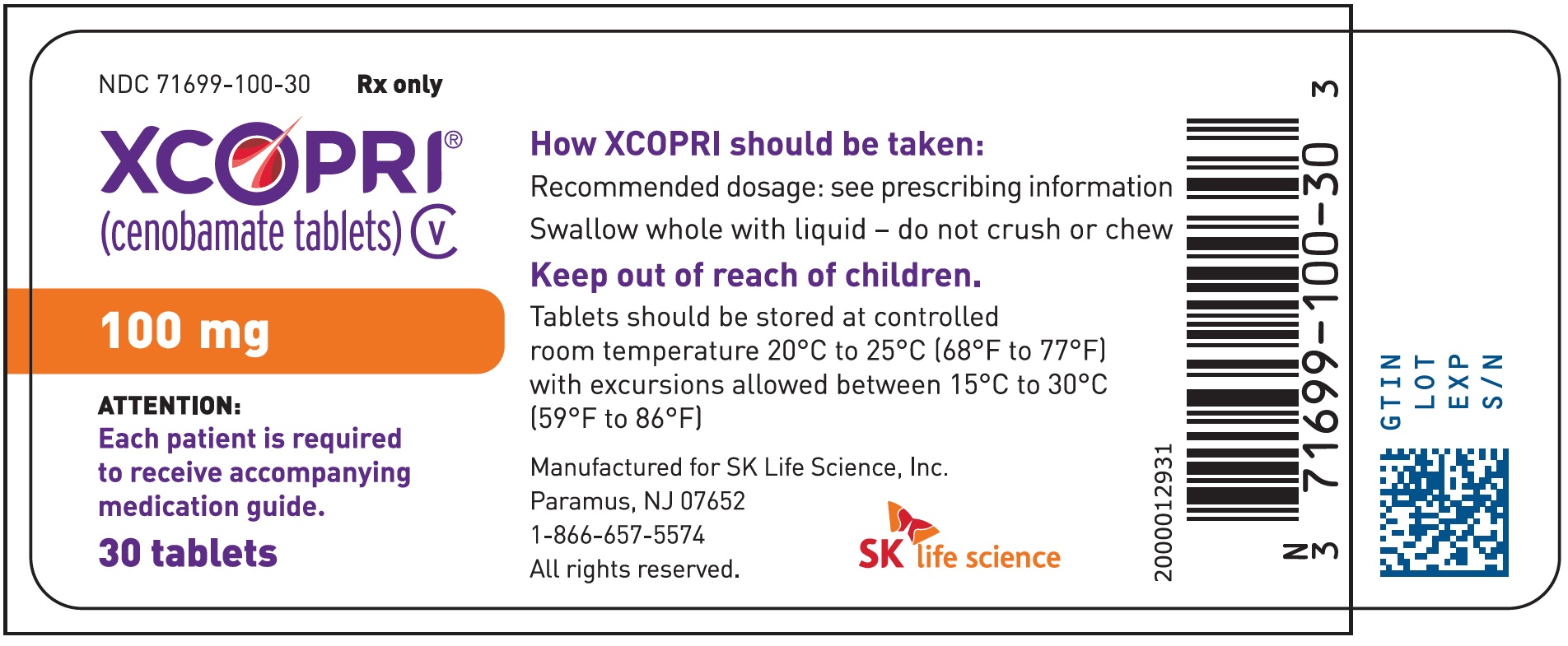
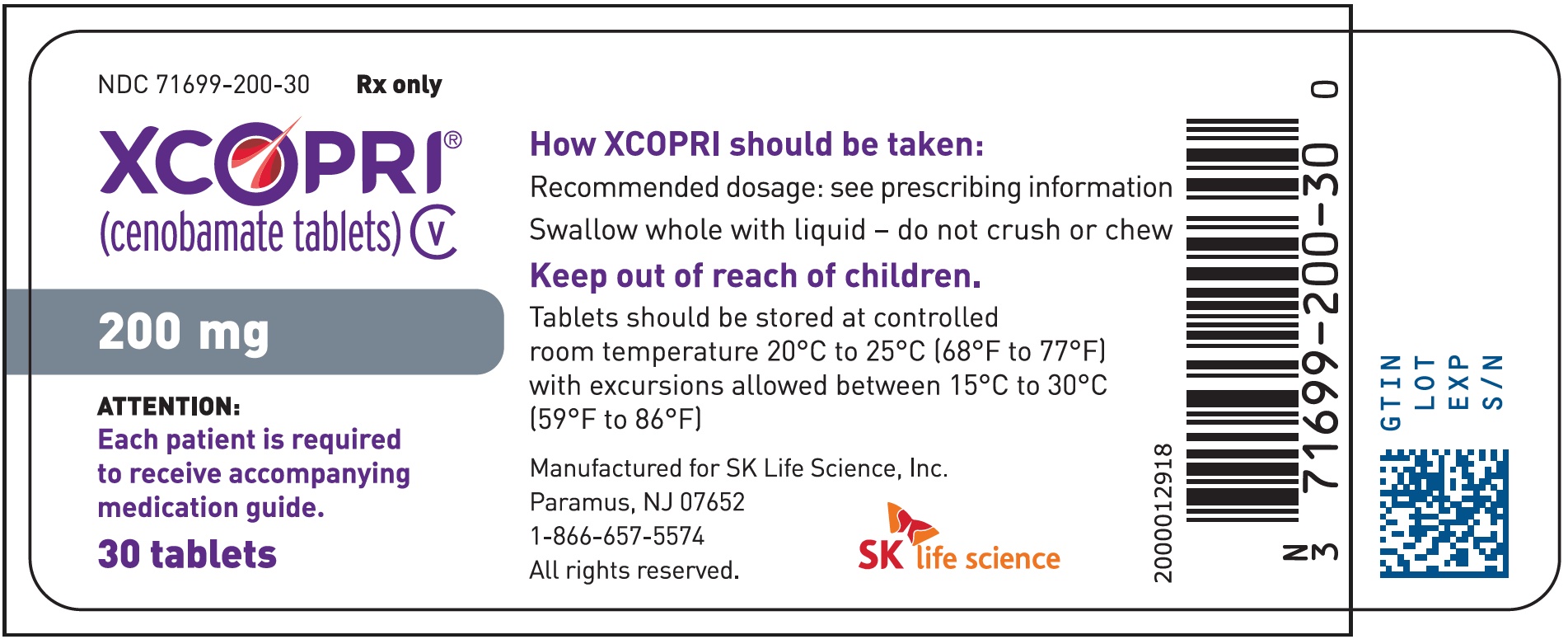
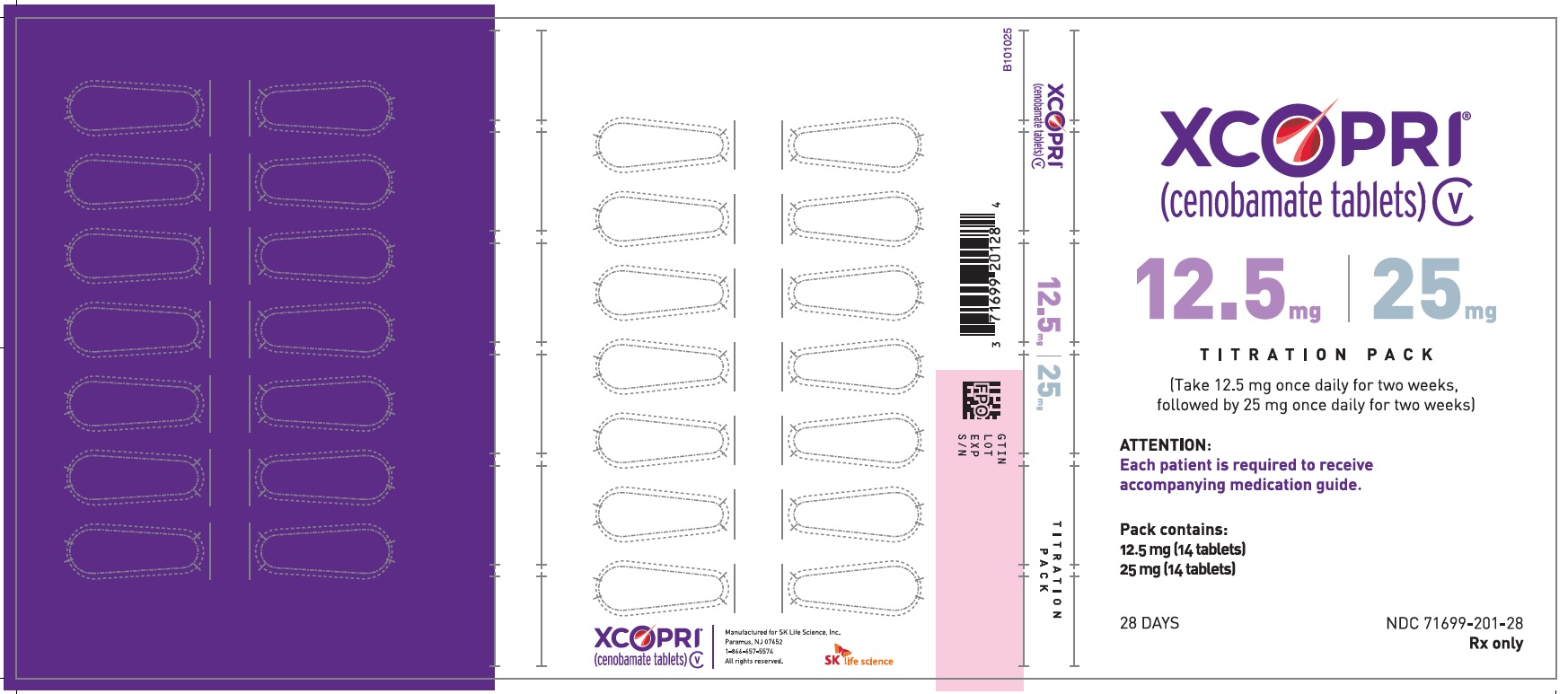
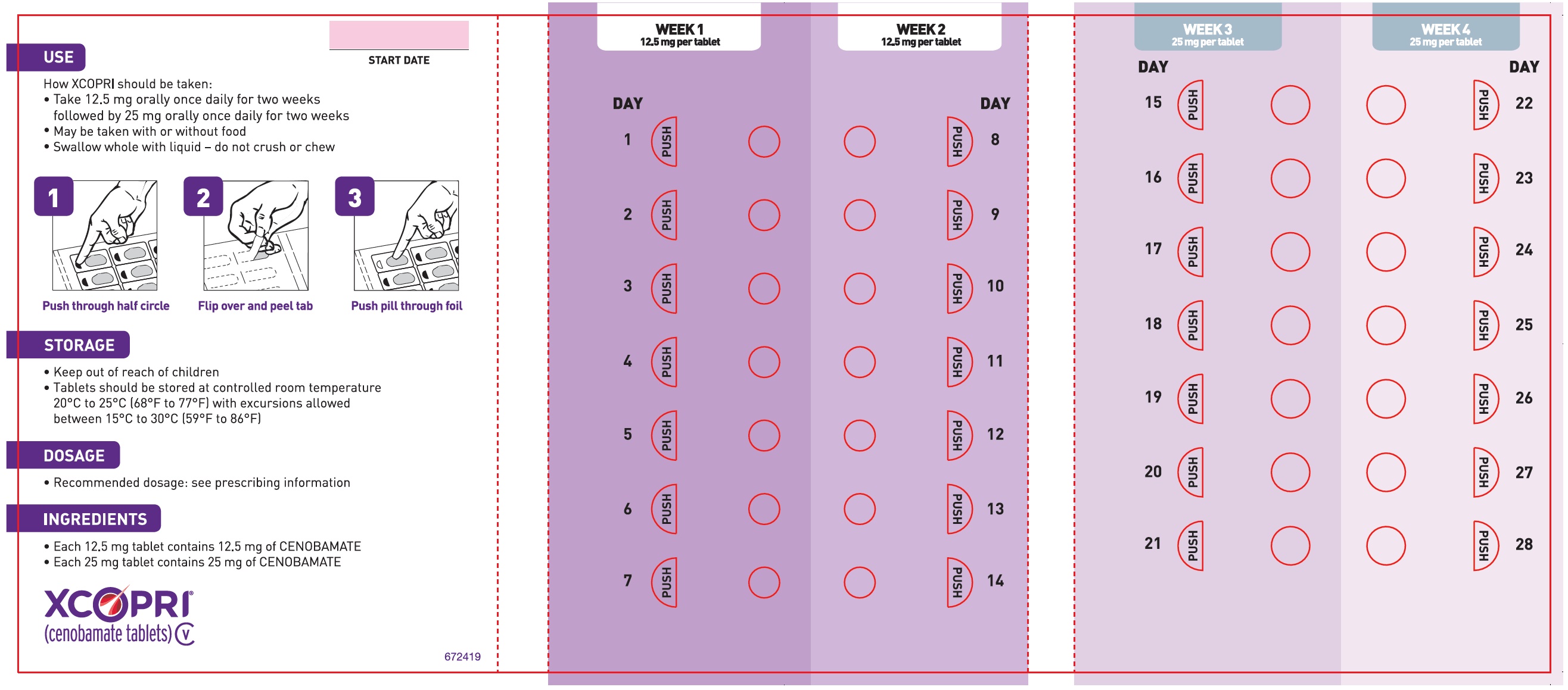
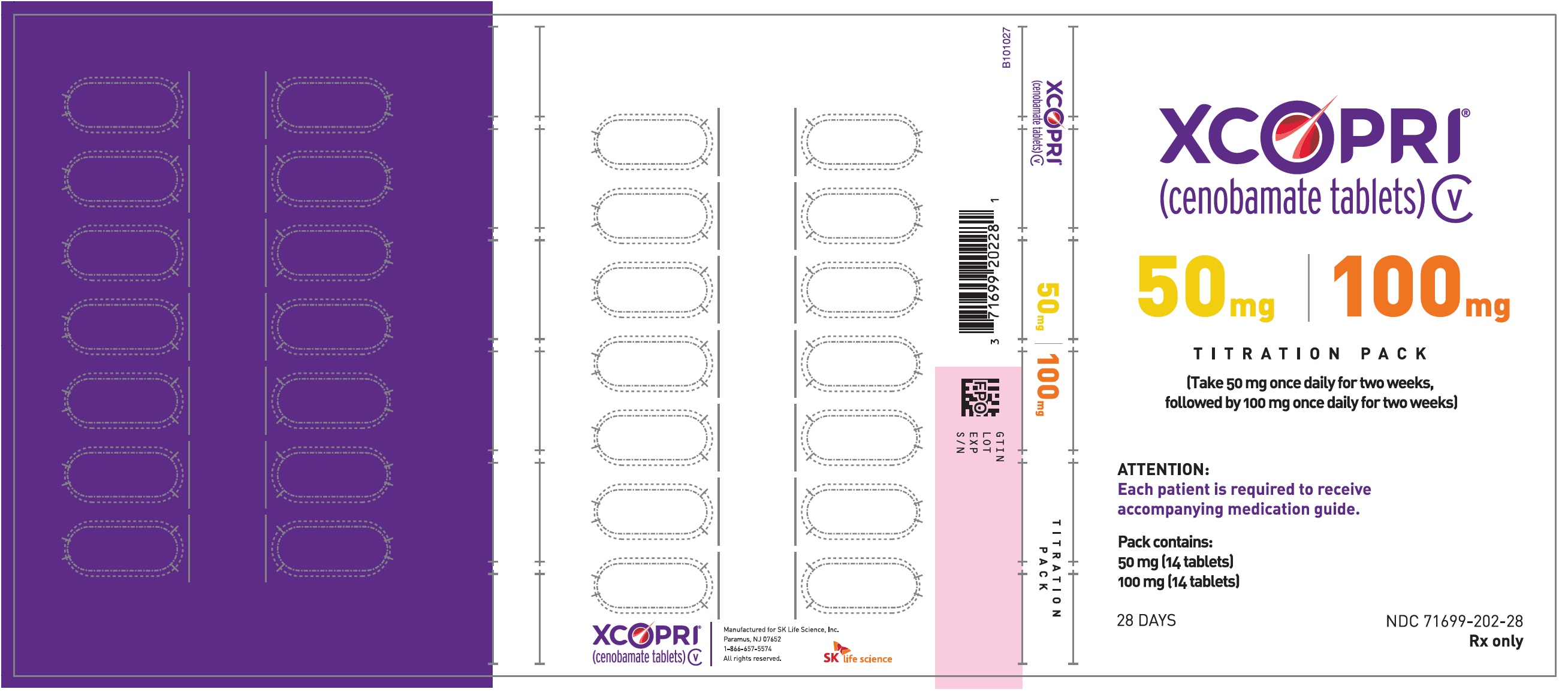
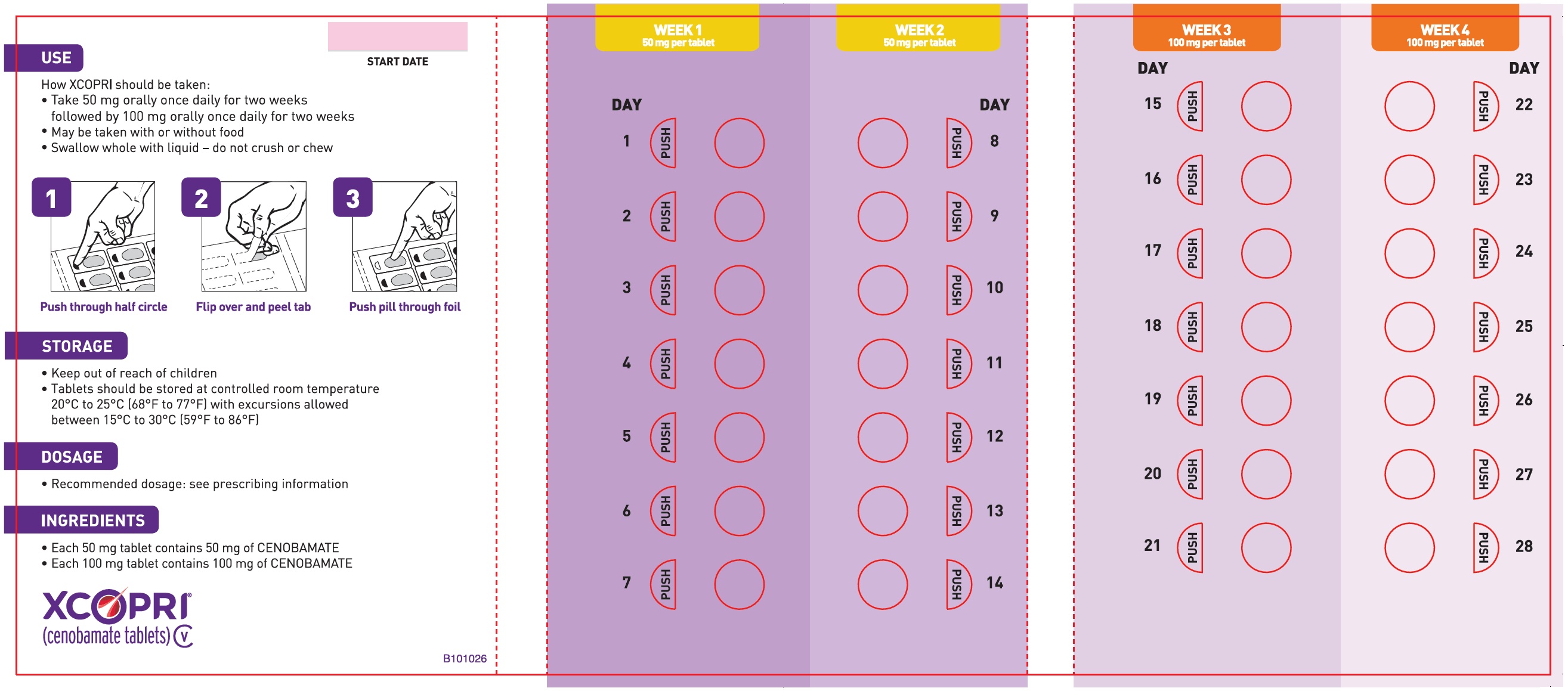
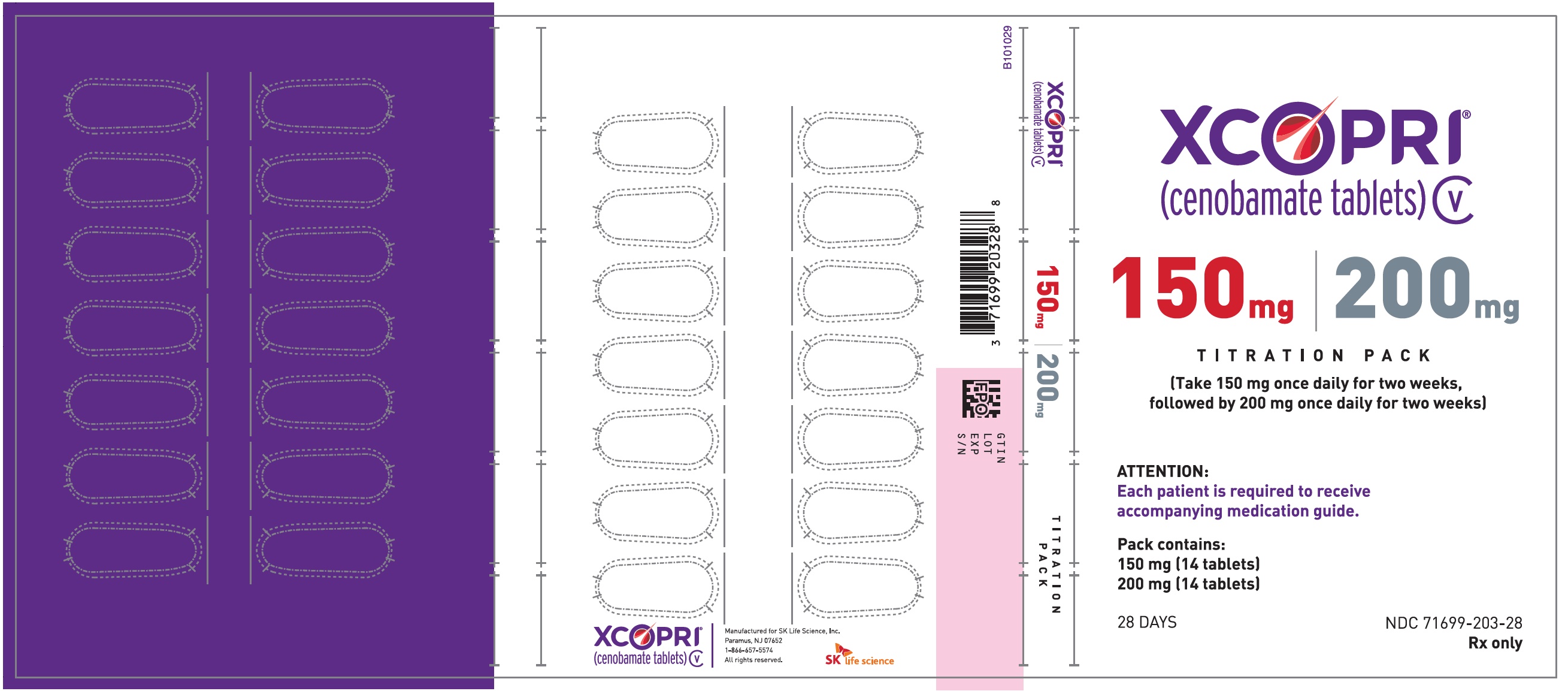
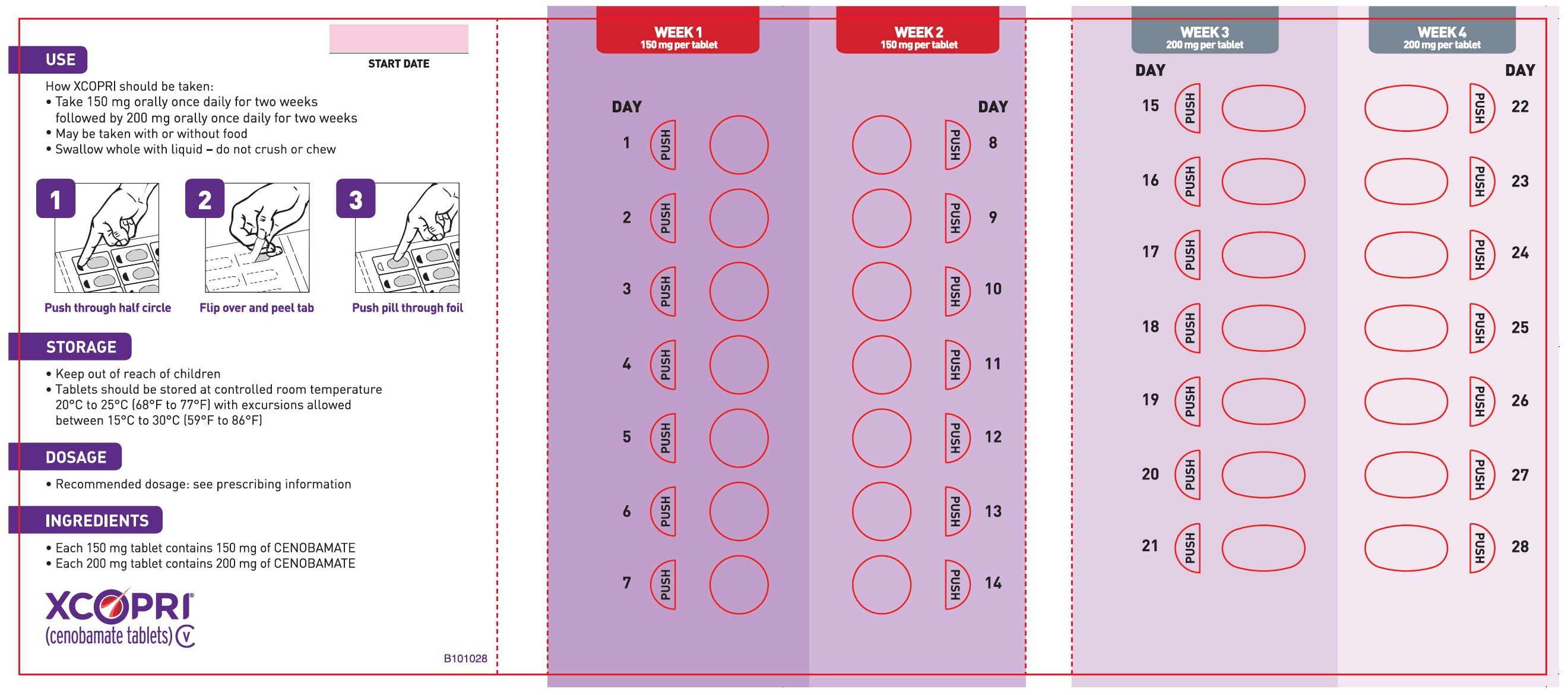
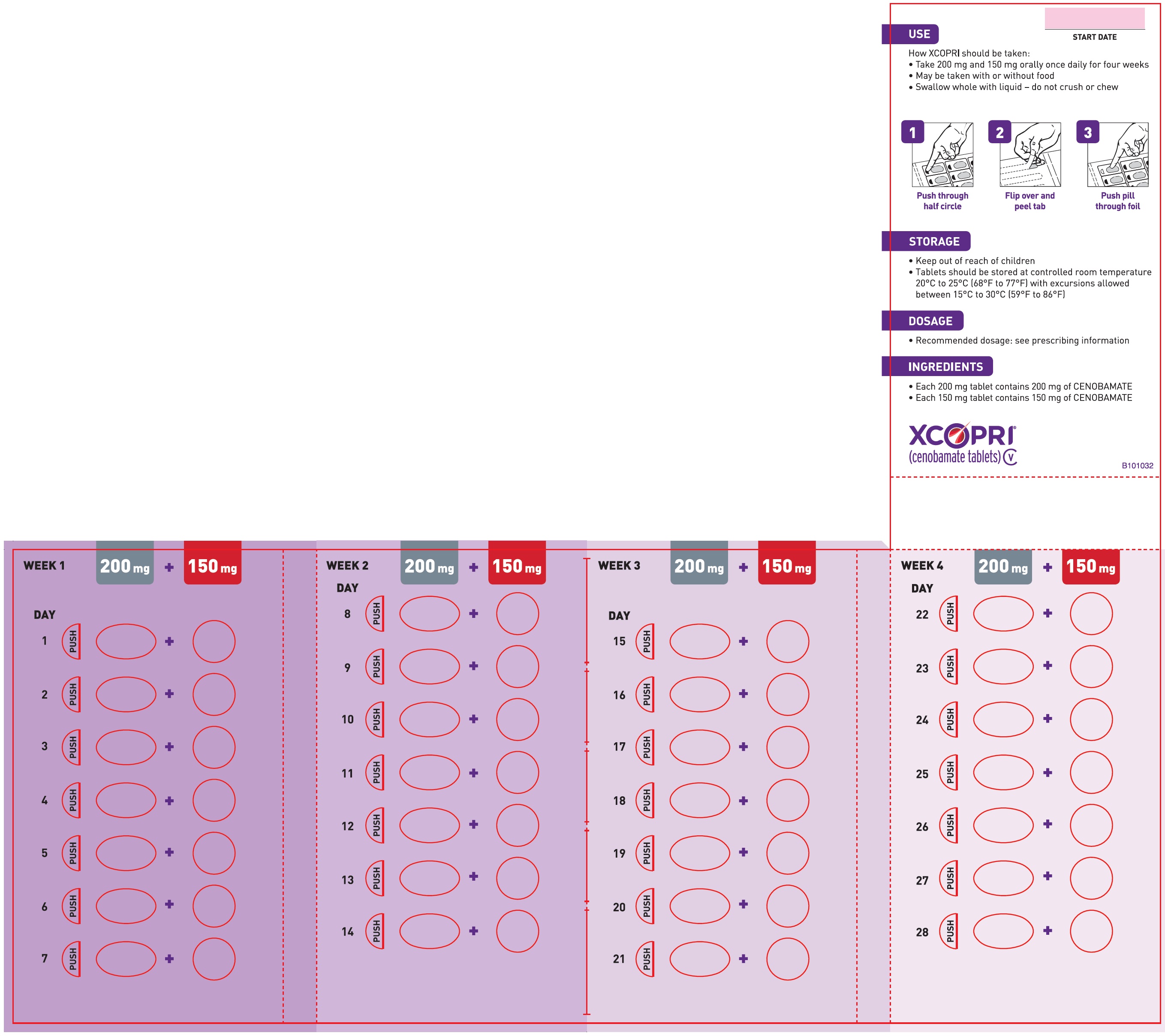
Dosing Instructions
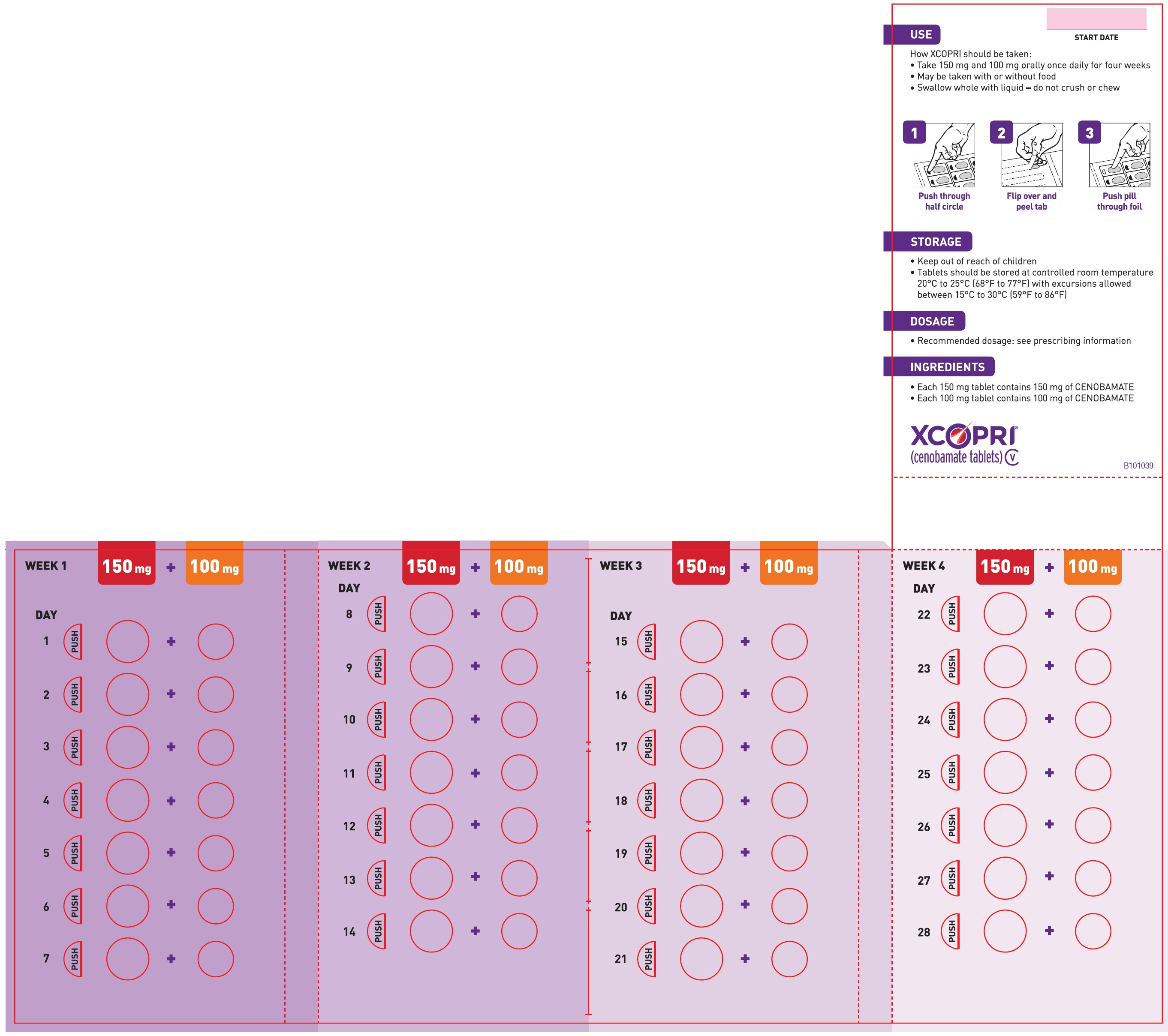
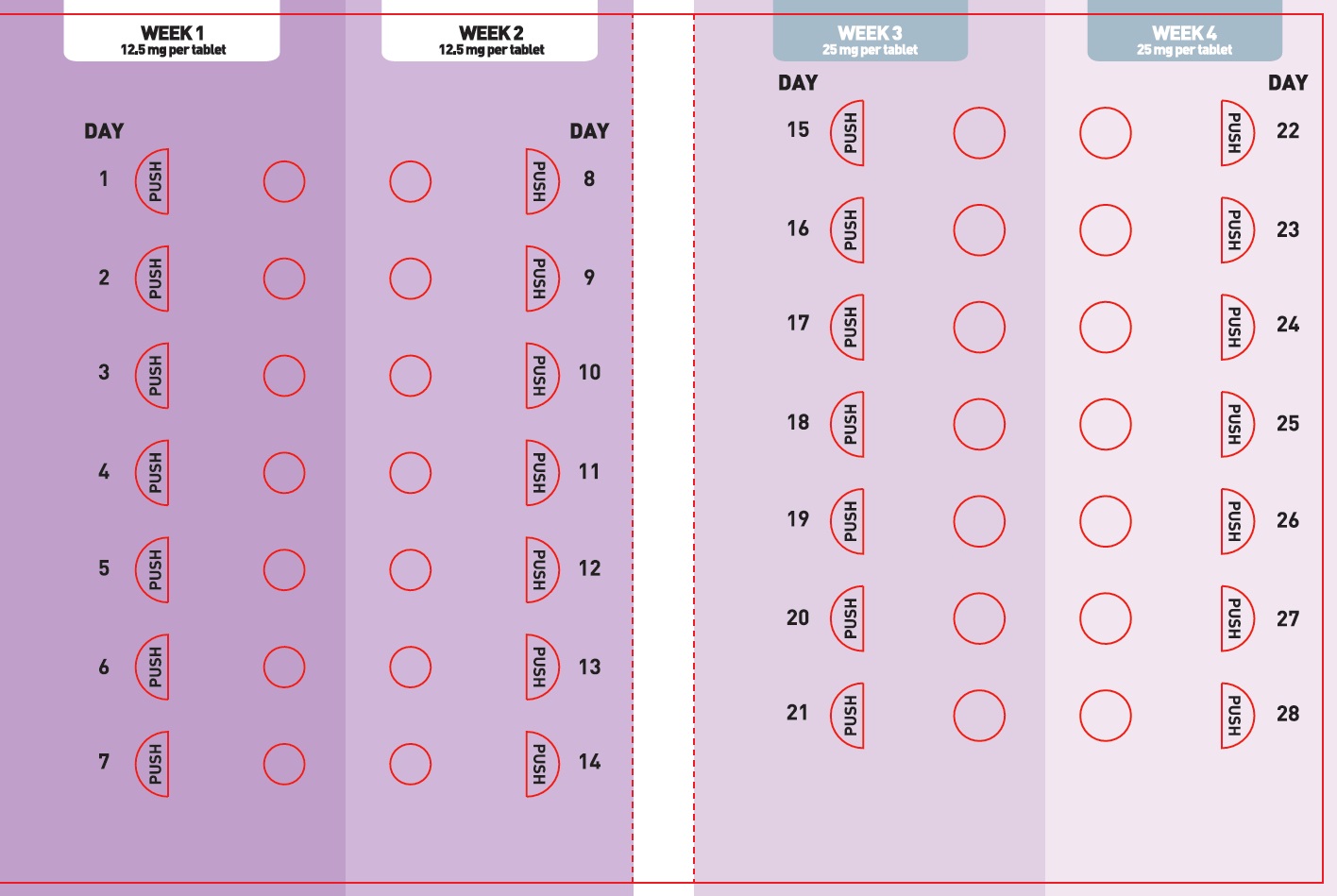
Counsel patients that XCOPRI may be taken any time with or without food. Instruct patients that XCOPRI tablets can be taken whole or crushed. The crushed tablet can be mixed with water and either administered by mouth as an oral suspension or administered via a nasogastric (NG) tube. Counsel patients administering XCOPRI as an oral suspension or via NG tube on appropriate preparation and administration instructions
Abuse and Dependence
Advise patients that XCOPRI is a federally controlled substance (CV) because it can be abused or lead to dependence
Manufactured for:
XCOPRI
For patent information: