Inlyta
What is Inlyta (Axitinib)?
Approved To Treat
Related Clinical Trials
Summary: The goal of this clinical research study is to find out if taking axitinib with ipilimumab is effective in treating advanced melanoma.
Summary: The ARON-1 Study is designed as an International Multicentric Retrospective Study to collect global experiences with the use of immuno-combinations in patients with metastatic RCC. Two Supplementary Studies (ARON-1α and ARON-1β) have been designed. The ARON-1α Supplementary Study has been designed to investigate for the presence of genomic signatures from tumor samples of patients treated with fir...
Summary: The purpose of this trial is to study the safety and effectiveness of OTX-TKI (axitinib intravitreal hydrogel) for the treatment of Non-Proliferative Diabetic Retinopathy. OTX-TKI is an intravitreal hydrogel embedded with axitinib. When the OTX-TKI hydrogel is administered into the vitreous cavity of the eye, the hydrogel begins to slowly break down, which allows the axitinib to be slowly released...
Related Latest Advances
Brand Information
- 1 mg tablets of INLYTA: red, film-coated, oval tablets, debossed with "Pfizer" on one side and "1 XNB" on the other side.
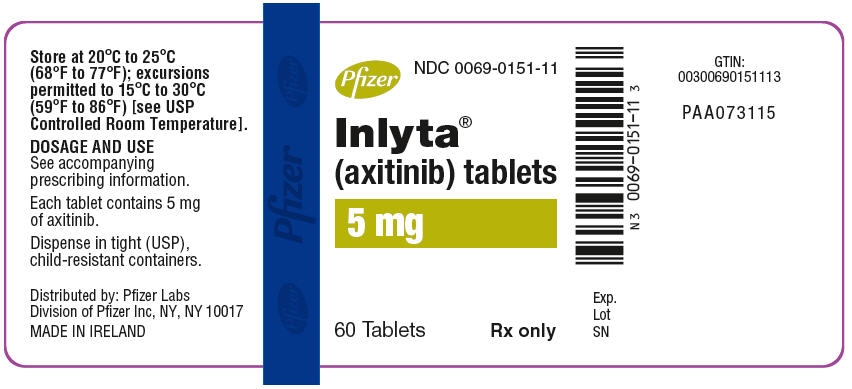
- 5 mg tablets of INLYTA: red, film-coated, triangular tablets, debossed with "Pfizer" on one side and "5 XNB" on the other side.
- Hypertension
- Arterial thromboembolic events
- Venous thromboembolic events
- Hemorrhage
- Cardiac failure
- Gastrointestinal perforation and fistula formation
- Thyroid dysfunction
- Reversible posterior leukoencephalopathy syndrome
- Proteinuria
- Hepatotoxicity
- Hepatic impairment
- 1 mg tablets are red film-coated, oval tablets debossed with "Pfizer" on one side and "1 XNB" on the other; available in bottles of 180: NDC 0069-0145-01.
- 5 mg tablets are red film-coated, triangular tablets debossed with "Pfizer" on one side and "5 XNB" on the other; available in bottles of 60: NDC 0069-0151-11.
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
(axitinib) tablets

(axitinib) tablets