Brand Name
Adefovir Dipivoxil
View Brand InformationFDA approval date: September 03, 2013
Classification: Hepatitis B Virus Nucleoside Analog Reverse Transcriptase Inhibitor
Form: Tablet
What is Adefovir Dipivoxil?
Adefovir dipivoxil tablets are indicated for the treatment of chronic hepatitis B in patients 12 years of age and older with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases or histologically active disease. This indication is based on histological, virological, biochemical, and serological responses in adult patients with HBeAg+ and HBeAg- chronic hepatitis B with compensated liver function, and with clinical evidence of lamivudine-resistant hepatitis B virus with either compensated or decompensated liver function. For patients 12 to less than 18 years of age, the indication is based on virological and biochemical responses in patients with HBeAg+ chronic hepatitis B virus infection with compensated liver function. Adefovir dipivoxil tablets are a nucleotide analogue indicated for the treatment of chronic hepatitis B in patients 12 years of age and older.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
ADEFOVIR DIPIVOXIL (ADEFOVIR DIPIVOXIL)
WARNING: SEVERE ACUTE EXACERBATIONS OF HEPATITIS, NEPHROTOXICITY, HIV RESISTANCE, LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY WITH STEATOSIS
Severe acute exacerbations of hepatitis have been reported in patients who have discontinued anti-Hepatitis B therapy including Adefovir Dipivoxil Tablets. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue anti-Hepatitis B therapy. If appropriate, resumption of anti-Hepatitis B therapy may be warranted
In patients at risk of or having underlying renal dysfunction, chronic administration of Adefovir Dipivoxil Tablets may result in nephrotoxicity. These patients should be monitored closely for renal function and may require dose adjustment
HIV resistance may emerge in chronic hepatitis B patients with unrecognized or untreated Human Immunodeficiency Virus (HIV) infection treated with antihepatitis B therapies, such as therapy with Adefovir Dipivoxil Tablets, that may have activity against HIV
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs alone or in combination with other antiretrovirals
1INDICATIONS AND USAGE
Adefovir Dipivoxil Tablets are indicated for the treatment of chronic hepatitis B in patients 12 years of age and older with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease.
This indication is based on histological, virological, biochemical, and serological responses in adult patients with HBeAg+ and HBeAg- chronic hepatitis B with compensated liver function, and with clinical evidence of lamivudine-resistant hepatitis B virus with either compensated or decompensated liver function.
For patients 12 to less than 18 years of age, the indication is based on virological and biochemical responses in patients with HBeAg+ chronic hepatitis B virus infection with compensated liver function.
2DOSAGE FORMS AND STRENGTHS
Adefovir Dipivoxil is available as tablets. Each tablet contains 10 mg of adefovir dipivoxil. The tablets are white, round, flat faced beveled edged tablets, debossed Σ 3 on one side and plain on the other side.
3CONTRAINDICATIONS
Adefovir Dipivoxil Tablets are contraindicated in patients with previously demonstrated hypersensitivity to any of the components of the product.
4ADVERSE REACTIONS
The following adverse reactions are discussed in other sections of the labeling:
- Severe acute exacerbations of Hepatitis
- Nephrotoxicity
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical and laboratory evidence of exacerbations of hepatitis have occurred after discontinuation of treatment with Adefovir Dipivoxil Tablets.
Adverse reactions to Adefovir Dipivoxil Tablets identified from placebo-controlled and open label studies include the following: asthenia, headache, abdominal pain, diarrhea, nausea, dyspepsia, flatulence, increased creatinine, and hypophosphatemia.
The incidence of these adverse reactions in studies 437 and 438, where 522 patients with chronic hepatitis B and compensated liver disease received double-blind treatment with Adefovir Dipivoxil Tablets (N=294) or placebo (N=228) for 48 weeks is presented in Table 2. Patients who received open-label Adefovir Dipivoxil Tablets for up to 240 weeks in Study 438 reported adverse reactions similar in nature and severity to those reported in the first 48 weeks.
a In these studies, the overall incidence of adverse reactions with Adefovir Dipivoxil Tablets was similar to that reported with placebo. The incidence of adverse reactions is derived from treatment-related events as identified by the study investigators.
No patients treated with Adefovir Dipivoxil Tablets developed a confirmed serum creatinine increase greater than or equal to 0.5 mg/dL from baseline or confirmed phosphorus decrease to 2 mg/dL or less by Week 48. By Week 96, 2% of Adefovir Dipivoxil Tablets-treated patients, by Kaplan-Meier estimate, had increases in serum creatinine greater than or equal to 0.5 mg/dL from baseline (no placebo-controlled results were available for comparison beyond Week 48). For patients who chose to continue Adefovir Dipivoxil Tablets for up to 240 weeks in Study 438, 4 of 125 patients (3%) had a confirmed increase of 0.5 mg/dL from baseline. The creatinine elevation resolved in 1 patient who permanently discontinued treatment and remained stable in 3 patients who continued treatment. For 65 patients who chose to continue Adefovir Dipivoxil Tablets for up to 240 weeks in Study 437, 6 had a confirmed increase in serum creatinine of greater than or equal to 0.5 mg/dL from baseline with 2 patients discontinuing from the study due to the elevated serum creatinine concentration.
4.2Special Risk Patients
Pre- and Post-Liver Transplantation Patients
Additional adverse reactions observed from an open-label study (Study 435) in pre- and post- liver transplantation patients with chronic hepatitis B and lamivudine-resistant hepatitis B administered Adefovir Dipivoxil Tablets once daily for up to 203 weeks include: abnormal renal function, renal failure, vomiting, rash, and pruritus.
Changes in renal function occurred in pre-and post-liver transplantation patients with risk factors for renal dysfunction, including concomitant use of cyclosporine and tacrolimus, renal insufficiency at baseline, hypertension, diabetes, and on-study transplantation. Therefore, the contributory role of Adefovir Dipivoxil Tablets to these changes in renal function is difficult to assess.
Increases in serum creatinine greater than or equal to 0.3 mg/dL from baseline were observed in 37% and 53% of pre-liver transplantation patients by Weeks 48 and 96, respectively, by Kaplan-Meier estimates. Increases in serum creatinine greater than or equal to 0.3 mg/dL from baseline were observed in 32% and 51% of post-liver transplantation patients by Weeks 48 and 96, respectively, by Kaplan-Meier estimates. Serum phosphorus values less than 2 mg/dL were observed in 3/226 (1.3%) of pre-liver transplantation patients and in 6/241 (2.5%) of post-liver transplantation patients by last study visit. Four percent (19 of 467) of patients discontinued treatment with Adefovir Dipivoxil Tablets due to renal adverse events.
4.3Pediatric Patients
Assessment of adverse reactions is based on a placebo-controlled study (Study 518) in which 173 pediatric patients aged 2 to less than 18 years with chronic hepatitis B and compensated liver disease received double-blind treatment with Adefovir Dipivoxil Tablets (N=115), or placebo (N=58) for 48 weeks
The safety profile of Adefovir Dipivoxil Tablets in patients 12 to less than 18 years of age (N=56) was similar to that observed in adults. No pediatric patients treated with Adefovir Dipivoxil Tablets developed a confirmed serum creatinine increase greater than or equal to 0.5 mg/dL from baseline or a confirmed phosphorus decrease to less than 2 mg/dL by Week 48.
4.4Post-Marketing Experience
In addition to adverse reaction reports from clinical trials, the following possible adverse reactions have also been identified during post-approval use of adefovir dipivoxil. Because these events have been reported voluntarily from a population of unknown size, estimates of frequency cannot be made.
Metabolism and Nutrition Disorders: hypophosphatemia
Gastrointestinal Disorders: pancreatitis
Musculoskeletal System and Connective Tissue Disorders: myopathy, osteomalacia (manifested as bone pain and may contribute to fractures), both associated with proximal renal tubulopathy
Renal and Urinary Disorders: renal failure, Fanconi syndrome, proximal renal tubulopathy
5DRUG INTERACTIONS
Since adefovir is eliminated by the kidney, coadministration of Adefovir Dipivoxil Tablets with drugs that reduce renal function or compete for active tubular secretion may increase serum concentrations of either adefovir and/or these coadministered drugs
Patients should be monitored closely for adverse events when Adefovir Dipivoxil Tablets are coadministered with drugs that are excreted renally or with other drugs known to affect renal function
Adefovir Dipivoxil Tablets should not be administered in combination with VIREAD
6OVERDOSAGE
Doses of adefovir dipivoxil 500 mg daily for 2 weeks and 250 mg daily for 12 weeks have been associated with gastrointestinal side effects. If overdose occurs the patient must be monitored for evidence of toxicity, and standard supportive treatment applied as necessary.
Following a 10 mg single dose of Adefovir Dipivoxil Tablets, a four-hour hemodialysis session removed approximately 35% of the adefovir dose.
7DESCRIPTION
Adefovir dipivoxil is a diester prodrug of adefovir. Adefovir is an acyclic nucleotide analog with activity against human hepatitis B virus (HBV).
The chemical name of adefovir dipivoxil is 9-[2-[[bis[(pivaloyloxy)methoxy]-phosphinyl]-methoxy]ethyl]adenine. It has a molecular formula of C
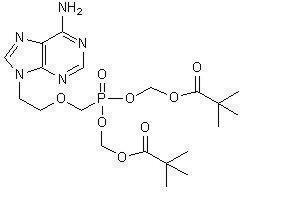
Adefovir dipivoxil is a white to off-white crystalline powder with an aqueous solubility of 19 mg/mL at pH 2.0 and 0.4 mg/mL at pH 7.2. It has an octanol/aqueous phosphate buffer (pH 7) partition coefficient (log p) of 1.91.
Adefovir Dipivoxil Tablets are for oral administration. Each tablet contains 10 mg of adefovir dipivoxil and the following inactive ingredients: copovidone, anhydrous lactose, microcrystalline cellulose, silicon dioxide, crospovidone and magnesium stearate.
8HOW SUPPLIED / STORAGE AND HANDLING
Adefovir Dipivoxil is available as tablets. Each tablet contains 10 mg of adefovir dipivoxil. The tablets are white, round, flat faced beveled edged tablets, debossed ∑3 on one side and plain on the other side. They are packaged as follows: Bottles of 30 tablets (NDC 42794-003-08) containing polyester and desiccant and closed with a child-resistant closure.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Inform patients of the potential risks and benefits of Adefovir Dipivoxil Tablets and of alternative modes of therapy.
- Instruct patients to:
-Follow a regular dosing schedule to avoid missing doses.
-Immediately report any severe abdominal pain, muscle pain, yellowing of the eyes, dark urine, pale stools, and/or loss in appetite.
-Inform their doctor or pharmacist if they develop any unusual symptom(s), or if any known symptom persists or worsens.
- Advise patients that:
- The optimal duration of Adefovir Dipivoxil Tablets treatment and the relationship between treatment response and long-term outcomes such as hepatocellular carcinoma or decompensated cirrhosis are not known.
- Patients should not discontinue Adefovir Dipivoxil Tablets without first informing their physician
- Routine laboratory monitoring and follow-up with a physician is important during Adefovir Dipivoxil Tablets therapy.
- Obtaining HIV antibody testing prior to starting Adefovir Dipivoxil Tablets is important
- Adefovir Dipivoxil Tablets should not be administered concurrently with ATRIPLA or COMPLERA or STRIBILD or TRUVADA or VIREAD
- Lamivudine-resistant patients should use Adefovir Dipivoxil Tablets in combination with lamivudine and not as Adefovir Dipivoxil Tablets monotherapy
- Inform patients that there is an antiretroviral pregnancy registry to monitor fetal outcomes of pregnant women exposed to Adefovir Dipivoxil
Manufactured by:
Sigmapharm Laboratories, LLC
Bensalem, PA 19020
OS003-09 REV.0821
10PRINCIPAL DISPLAY PANEL- Adefovir Dipivoxil 10 mg Bottle Label
Sigmapharm Laboratories, LLC
NDC 42794-003-08
Adefovir Dipivoxil Tablets
10 mg
PHARMACIST: DISPENSE THE PATIENT INFORMATION LEAFLET WITH DRUG PRODUCT
Rx Only
30 Tablets
11PRINCIPAL DISPLAY PANEL- Adefovir Dipivoxil 10 mg Carton Label
Sigmapharm Laboratories, LLC
NDC 42794-
Adefovir Dipivoxil Tablets
10 mg
PHARMACIST: DISPENSE THE PATIENT INFORMATION LEAFLET WITH DRUG PRODUCT
Rx Only
30 Tablets
