Brand Name
Ixempra
Generic Name
Ixabepilone
View Brand Information FDA approval date: October 16, 2007
Form: Kit
What is Ixempra (Ixabepilone)?
IXEMPRA is indicated in combination with capecitabine for the treatment of patients with metastatic or locally advanced breast cancer resistant to treatment with an anthracycline and a taxane, or whose cancer is taxane resistant and for whom further anthracycline therapy is contraindicated. Anthracycline resistance is defined as progression while on therapy or within 6 months in the adjuvant setting or 3 months in the metastatic setting. Taxane resistance is defined as progression while on therapy or within 12 months in the adjuvant setting or 4 months in the metastatic setting. IXEMPRA is indicated as monotherapy for the treatment of metastatic or locally advanced breast cancer in patients whose tumors are resistant or refractory to anthracyclines, taxanes, and capecitabine. IXEMPRA, a microtubule inhibitor, in combination with capecitabine is indicated for the treatment of metastatic or locally advanced breast cancer in patients after failure of an anthracycline and a taxane . IXEMPRA as monotherapy is indicated for the treatment of metastatic or locally advanced breast cancer in patients after failure of an anthracycline, a taxane, and capecitabine .
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
IXEMPRA (ixabepilone)
WARNING: TOXICITY IN PATIENTS WITH HEPATIC IMPAIRMENT
IXEMPRA in combination with capecitabine is contraindicated in patients with AST or ALT >2.5 x ULN or bilirubin >1 x ULN due to increased risk of toxicity and neutropenia-related death [see Contraindications (
1INDICATIONS AND USAGE
IXEMPRA is indicated in combination with capecitabine for the treatment of patients with metastatic or locally advanced breast cancer resistant to treatment with an anthracycline and a taxane, or whose cancer is taxane resistant and for whom further anthracycline therapy is contraindicated. Anthracycline resistance is defined as progression while on therapy or within 6 months in the adjuvant setting or 3 months in the metastatic setting. Taxane resistance is defined as progression while on therapy or within 12 months in the adjuvant setting or 4 months in the metastatic setting [see Clinical Studies (
IXEMPRA is indicated as a single agent for the treatment of patients with metastatic or locally advanced breast cancer whose tumors are resistant or refractory to anthracyclines, taxanes, and capecitabine [see Clinical Studies (
2DOSAGE FORMS AND STRENGTHS
IXEMPRA for injection, 15 mg single-dose vial supplied with DILUENT for IXEMPRA, 8 mL.
IXEMPRA for injection, 45 mg single-dose vial supplied with DILUENT for IXEMPRA, 23.5 mL.
3CONTRAINDICATIONS
IXEMPRA is contraindicated in patients who have:
- a neutrophil count <1500 cells/mm
- a history of severe hypersensitivity to agents containing Cremophor
IXEMPRA in combination with capecitabine is contraindicated in patients with AST or ALT >2.5 x ULN or bilirubin >1 x ULN
4ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections.
- Peripheral neuropathy
- Myelosuppression
- Hypersensitivity reactions
- Cardiac Adverse reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in other clinical trials and may not reflect the rates observed in clinical practice.
Unless otherwise specified, assessment of adverse reactions is based on one randomized study (Study 046) and one single-arm study (Study 081). In Study 046, 369 patients with metastatic breast cancer were treated with IXEMPRA 40 mg/m
The most common adverse reactions (≥20%) reported by patients receiving IXEMPRA were peripheral sensory neuropathy, fatigue/asthenia, myalgia/arthralgia, alopecia, nausea, vomiting, stomatitis/mucositis, diarrhea, and musculoskeletal pain. The following additional reactions occurred in ≥20% in combination treatment: palmar-plantar erythrodysesthesia (hand-foot) syndrome, anorexia, abdominal pain, nail disorder, and constipation. The most common hematologic abnormalities (>40%) include neutropenia, leukopenia, anemia, and thrombocytopenia.
Table 4 presents nonhematologic adverse reactions reported in 5% or more of patients. Hematologic abnormalities are presented separately in Table 5.
4.2Postmarketing Experience
The following adverse reaction has been identified during postapproval use of IXEMPRA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
Procedural Complications: Radiation recall
5OVERDOSAGE
In patients who received an overdosage of IXEMPRA of up to 100 mg/m
There is no known antidote for overdosage of IXEMPRA. In case of overdosage, closely monitor patients for adverse reactions and provide supportive treatment as clinically indicated.
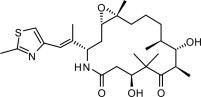
6DESCRIPTION
IXEMPRA (ixabepilone) is a microtubule inhibitor belonging to a class of antineoplastic agents, the epothilones and their analogs. The epothilones are isolated from the myxobacterium
The chemical name for ixabepilone is (1
IXEMPRA (ixabepilone) for injection is intended for intravenous infusion only after constitution with the supplied DILUENT and after further dilution with a specified infusion fluid
7REFERENCES
- OSHA Hazardous Drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html OSHA
8HOW SUPPLIED/STORAGE AND HANDLING
IXEMPRA is supplied as a
IXEMPRA
IXEMPRA is a hazardous drug. Follow applicable special handling and disposal procedures.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling
9.1Peripheral Neuropathy
Advice patients to report any numbness and tingling of the hands or feet to their healthcare provider
9.2Fever/Neutropenia
Instruct patients to immediately contact their healthcare provider if a fever of 100.5° F or greater or other evidence of potential infection such as chills, cough, or burning or pain on urination occur
9.3Hypersensitivity Reactions
Advise patients to immediately contact their healthcare provider if they experience urticaria, pruritus, rash, flushing, swelling, dyspnea, chest tightness, or other hypersensitivity-related symptoms following an infusion of IXEMPRA
9.4Cardiac Adverse Reactions
Advise patients to immediately contact call their healthcare provider if they experience chest pain, difficulty breathing, palpitations, or unusual weight gain
9.5Embryo-Fetal Toxicity
Advise females of reproductive potential and pregnant women of the potential risk to a fetus. Advise patients to inform their healthcare provider of a known or suspected pregnancy.
Advise females of reproductive potential to use effective contraception during treatment with IXEMPRA and for 7 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with IXEMPRA and for 4 months after the last dose
9.6Lactation
Advise women not to breastfeed during treatment with IXEMPRA and for 2 weeks after the last dose [see Use in Specific Populations (
9.7Infertility
Advise males and females of reproductive potential that IXEMPRA may impair fertility [see Use in Specific Populations (
9.8Alcohol Content in IXEMPRA
Explain to patients the possible effects of the alcohol content in IXEMPRA, including possible effects on the central nervous system [see Warnings and Precautions (

