Enhertu
What is Enhertu (Fam-Trastuzumab Deruxtecan-Nxki)?
Top Global Experts
Related Clinical Trials
Summary: This clinical trial is designed to assess the efficacy and safety of the triplet combination of trastuzumab deruxtecan (ENHERTU, T-DXd, DS-8201a) plus a fluoropyrimidine plus pembrolizumab versus standard of care (SoC) chemotherapy plus trastuzumab plus pembrolizumab as first-line therapy in participants with unresectable, locally advanced or metastatic HER2-positive tumor PD-L1 CPS ≥1 gastric or ...
Summary: This clinical trial is designed to assess the efficacy and safety of trastuzumab deruxtecan (T-DXd; Enhertu®) in combination with pembrolizumab versus platinum-based chemotherapy in combination with pembrolizumab in participants with no prior therapy for locally advanced unresectable or metastatic non-squamous NSCLC, whose tumors have HER2-overexpressing and PD-L1 TPS \<50% without known AGA that ...
Summary: The goal of this study is to test the efficacy of using a 12-week, home-based, unsupervised aerobic and resistance training exercise program for changes in cancer-related fatigue in patients with metastatic breast cancer who are receiving Enhertu.
Related Latest Advances
Brand Information
- Interstitial Lung Disease (ILD) and pneumonitis, including fatal cases, have been reported with ENHERTU. Monitor for and promptly investigate signs and symptoms including cough, dyspnea, fever, and other new or worsening respiratory symptoms. Permanently discontinue ENHERTU in all patients with Grade 2 or higher ILD/pneumonitis. Advise patients of the risk and the need to immediately report symptoms
- Embryo-Fetal Toxicity: Exposure to ENHERTU during pregnancy can cause embryo-fetal harm. Advise patients of these risks and the need for effective contraception
- Interstitial Lung Disease/Pneumonitis
- Neutropenia
- Left Ventricular Dysfunction
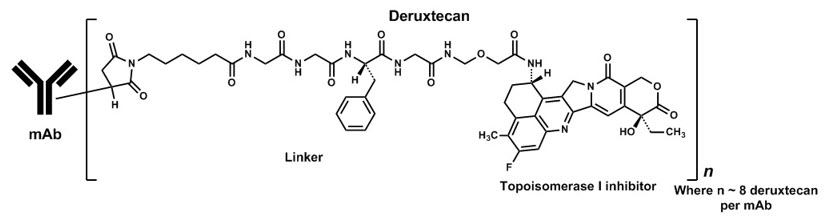
- OSHA Hazardous Drugs.