Brand Name
Zymaxid
Generic Name
Gatifloxacin
View Brand Information FDA approval date: May 19, 2010
Classification: Quinolone Antimicrobial
Form: Solution
What is Zymaxid (Gatifloxacin)?
ZYMAXID ® is indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the following organisms: Aerobic gram-positive bacteria: Staphylococcus aureus Staphylococcus epidermidis Streptococcus mitis group * Streptococcus oralis * Streptococcus pneumoniae Aerobic gram-negative bacteria: Haemophilus influenzae *Efficacy for these organisms were studied in fewer than 10 infections. ZYMAXID ® is a quinolone antimicrobial indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the following organisms: Haemophilus influenzae, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus mitis group, Streptococcus oralis, Streptococcus pneumoniae
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
ZYMAXID (gatifloxacin)
1INDICATIONS AND USAGE
ZYMAXID
- Aerobic gram-positive bacteria:
- Aerobic gram-negative bacteria:
*Efficacy for these organisms were studied in fewer than 10 infections
2DOSAGE AND ADMINISTRATION
- Day 1: Instill one drop every two hours in the affected eye(s) while awake, up to 8 times.
- Day 2 through Day 7: Instill one drop two to four times daily in the affected eye(s) while awake.
3DOSAGE FORMS AND STRENGTHS
Ophthalmic solution: 0.5% gatifloxacin (5 mg/mL)
4CONTRAINDICATIONS
ZYMAXID
5ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Hypersensitivity [
- Growth of Resistant Organisms With Prolonged Use [
- Corneal Endothelial Cell Injury [
5.1Clinical StudiesExperience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In clinical studies of patients with bacterial conjunctivitis treated with ZYMAXID
Additional adverse reactions reported with other formulations of gatifloxacin ophthalmic solution in other clinical studies included chemosis, conjunctival hemorrhage, dry eye, eye discharge, eyelid edema, headache, increased lacrimation, keratitis, red eye, papillary conjunctivitis, and reduced visual acuity.
5.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ZYMAXID
6DESCRIPTION
ZYMAXID
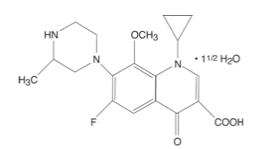
ZYMAXID
ZYMAXID
7CLINICAL STUDIES
In two randomized, double-masked, multicenter clinical trials, where patients 1-89 years of age were dosed for 5 days, ZYMAXID
8HOW SUPPLIED/STORAGE AND HANDLING
ZYMAXID
2.5 mL in 5 mL bottle: NDC 0023-3615-25
Storage:Store at 15°-25°C (59°-77°F). Protect from freezing.
9PATIENT COUNSELING INFORMATION
Avoiding Contamination of the Product
Instruct patients to avoid contaminating the applicator tip with material from the eye, fingers, or other source.
Instruct patients to avoid contaminating the applicator tip with material from the eye, fingers, or other source.
Potential for Hypersensitivity Reactions
Advise patients to discontinue use immediately and contact the physician at the first sign of a rash or hypersensitivity reaction [see Warnings and Precautions (5.1) and Contraindication (4)].
Advise patients to discontinue use immediately and contact the physician at the first sign of a rash or hypersensitivity reaction [see Warnings and Precautions (5.1) and Contraindication (4)].
© 2017 Allergan. All rights reserved.
All trademarks are the property of their respective owners.
Patented. See www.allergan.com/patents
72366US14