Liraglutide
What is Victoza (Liraglutide)?
Managing type 2 diabetes isn’t just about numbers on a glucose monitor, it’s about living life with more stability, energy, and confidence. For many adults, controlling blood sugar can be a daily challenge, especially when diet and exercise alone aren’t enough. Victoza (liraglutide) is a medication designed to help people with type 2 diabetes take back control of their health by improving blood sugar management and supporting overall metabolic balance.
Victoza is a prescription injectable medication belonging to a class of drugs called GLP-1 receptor agonists (glucagon-like peptide-1 receptor agonists). It works by mimicking a natural hormone that helps regulate blood sugar, insulin levels, and appetite. Approved by the U.S. Food and Drug Administration (FDA) in 2010, Victoza has become a trusted, long-term therapy for adults with type 2 diabetes. Victoza has also shown heart health benefits in certain patients.
What does Victoza do?
Victoza is primarily used to improve blood sugar control in adults with type 2 diabetes mellitus, alongside diet and exercise. It helps the body lower blood sugar after meals and maintain steadier glucose levels throughout the day.
By improving how the body responds to food, Victoza helps reduce common diabetes symptoms such as fatigue, thirst, and frequent urination. Many patients also experience gradual weight loss, which can further support blood sugar management and reduce the risk of diabetes complications.
In clinical studies, patients taking Victoza achieved significant reductions in hemoglobin A1C (HbA1c), a key measure of long-term blood sugar control (NIH, 2024). Additionally, Victoza has been shown to lower the risk of major cardiovascular events, including heart attack and stroke, in adults with type 2 diabetes who have established heart disease (FDA, 2023).
Victoza is not intended to treat type 1 diabetes or diabetic ketoacidosis, and it should not be used as a substitute for insulin in patients who require insulin therapy.
How does Victoza work?
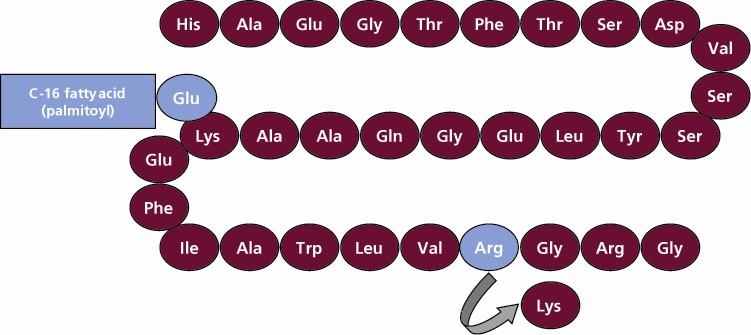
Victoza contains liraglutide, a synthetic version of a natural hormone called GLP-1 (glucagon-like peptide-1). GLP-1 plays a key role in controlling blood sugar levels, especially after eating.
Victoza works through several mechanisms:
- Stimulates insulin release: It helps the pancreas release more insulin when blood sugar levels rise after meals.
- Suppresses glucagon production: Glucagon is a hormone that raises blood sugar. Victoza reduces its release, preventing unnecessary spikes.
- Slows stomach emptying: By slowing digestion, it helps control how quickly sugar enters the bloodstream, reducing after-meal glucose surges.
- Reduces appetite: Many people taking Victoza feel fuller sooner, which can support weight loss and improve metabolic health.
Clinically, these effects help maintain smoother, more stable blood sugar levels throughout the day and night. This mechanism not only improves glucose control but also reduces strain on the pancreas and cardiovascular system, helping patients feel better and reduce long-term risks.
Victoza side effects
Like all prescription medications, Victoza can cause side effects, though many are mild and improve with continued use.
Common side effects include:
- Nausea or upset stomach (usually improves over time)
- Vomiting or diarrhea
- Loss of appetite
- Headache or fatigue
- Mild injection site reactions (such as redness or swelling)
Serious side effects (less common) include:
- Signs of pancreatitis (severe abdominal pain, nausea, or vomiting)
- Gallbladder problems
- Kidney issues (changes in urination or swelling)
- Possible thyroid tumors (rare, based on animal studies)
Patients should contact their healthcare provider immediately if they experience severe stomach pain, difficulty swallowing, shortness of breath, or a lump in the neck, as these could indicate serious complications.
Victoza is contraindicated in individuals with a personal or family history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia syndrome type 2 (MEN 2). Patients on other oral medications should consult their provider about timing due to slowed digestion. Regular monitoring of blood sugar, kidney function, and overall response is crucial for safety and efficacy.
Victoza dosage
Victoza is a once-daily subcutaneous injection (abdomen, thigh, or upper arm) that can be taken at any time, with or without food, as long as it’s consistent. Patients usually begin with a low dose to lessen stomach-related side effects, and the dose may be adjusted by a healthcare provider for optimal blood sugar control. Correct use of the multi-dose, prefilled Victoza pen is essential.
Patients should maintain their diabetes plan (diet, exercise, blood sugar checks). Physicians may order periodic HbA1c tests, and monitor weight and kidney function. Victoza is generally safe for older adults and those with mild kidney or liver impairment, though dose adjustments or closer monitoring may be needed.
Does Victoza have a generic version?
As of 2025, no generic version of Victoza (liraglutide) has been approved in the United States. The medication is currently manufactured and marketed by Novo Nordisk. However, international versions may exist in other markets.
Saxenda, a liraglutide formulation, is FDA-approved for chronic weight management in adults with obesity or overweight conditions, distinct from Victoza. When a generic Victoza becomes available, it must meet FDA standards for bioequivalence, safety, and effectiveness. Patients can explore cost-saving programs or insurance support.
Conclusion
Victoza (liraglutide) is a proven and effective treatment that helps adults with type 2 diabetes gain better control over their blood sugar and overall health. By mimicking a natural hormone, it not only stabilizes glucose levels but also supports weight management and cardiovascular protection in high-risk patients.
While side effects are possible, most are manageable and improve over time. Consistent use, regular follow-up, and open communication with your healthcare provider are key to Victoza’s success. It’s a powerful tool for a long-term, holistic approach to diabetes management, empowering patients to live more freely, confidently, and healthfully.
References
- U.S. Food and Drug Administration (FDA). (2023). Victoza (liraglutide) prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Liraglutide (injection route): Drug information. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Liraglutide injection: Uses, side effects, and precautions. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). GLP-1 receptor agonists and type 2 diabetes management. Retrieved from https://www.nih.gov
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- Liraglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures in both genders of rats and mice. It is unknown whether VICTOZA causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans, as the human relevance of liraglutide-induced rodent thyroid C-cell tumors has not been determined
- VICTOZA is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of VICTOZA and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with VICTOZA
- as an adjunct to diet and exercise to improve glycemic control in patients 10 years and older with type 2
- diabetes mellitus,
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial
- Medullary Thyroid Carcinoma
- Hypersensitivity
- Risk of Thyroid C-cell Tumors
- Pancreatitis
- Hypoglycemia
- Renal Impairment
- Hypersensitivity Reactions
- Acute Gallbladder Disease
- Medullary thyroid carcinoma
- Dehydration resulting from nausea, vomiting and diarrhea.
- Increased serum creatinine, acute renal failure or worsening of chronic renal failure, sometimes requiring hemodialysis.
- Angioedema and anaphylactic reactions.
- Allergic reactions: rash and pruritus
- Skin and subcutaneous tissue disorder: cutaneous amyloidosis
- Acute pancreatitis, hemorrhagic and necrotizing pancreatitis sometimes resulting in death
- Hepatobiliary disorders: hyperbilirubinemia, elevations of liver enzymes, cholestasis, hepatitis, cholecystitis, cholelithiasis requiring cholecystectomy
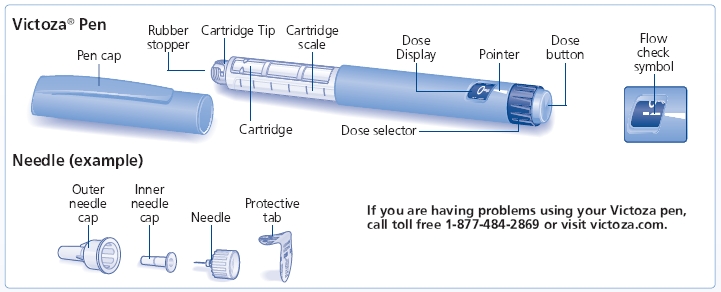
- Δ Always use a new needle for each injection to prevent contamination.
- Δ Always remove the needle after each injection, and store your pen without the needle
- attached. This reduces the risk of contamination, infection, leakage of liraglutide,
- blocked needles and inaccurate dosing.
- Δ Keep your Victoza pen and all medicines out of the reach of children.
- Δ If you drop your Victoza pen, repeat “First Time Use For Each New Pen” (steps A
- through D).
- Δ Be careful not to bend or damage the needle.
- Δ Do not use the cartridge scale to measure how much Victoza to inject.
- Δ Be careful when handling used needles to avoid needle stick injuries.
- Δ You can use your Victoza pen for up to 30 days after you use it the first time.
- Take your new Victoza pen out of the refrigerator.
- Wash hands with soap and water before use.
- Check pen label before each use to make sure it is your Victoza pen.
- Pull off pen cap (See Figure A).
- Check Victoza in the cartridge. The liquid should be clear, colorless and free of particles. If not, do not use.
- Wipe the rubber stopper with an alcohol swab.
- Remove protective tab from outer needle cap (See Figure B).
- Push outer needle cap containing the needle straight onto the pen, then screw needle on until secure.
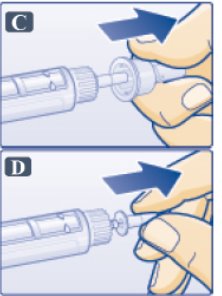
- Pull off outer needle cap (See Figure C). Do not throw away
- Pull off inner needle cap and throw away (See Figure D). A small drop of liquid may appear. This is normal.
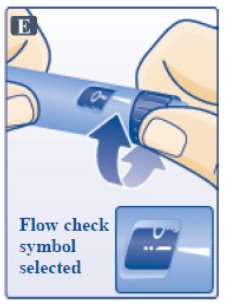
- Turn dose selector until flow check symbol (--) lines up with pointer (See Figure E). The flow check symbol does not administer the dose as prescribed by your healthcare provider.
- To select the dose prescribed by your healthcare provider, continue to Step G under “Routine Use”.
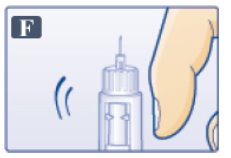
- Hold pen with needle pointing up.
- Tap cartridge gently with your finger a few times to bring any air bubbles to the top of the cartridge (See Figure F).
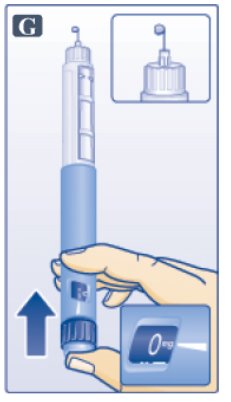
- Keep needle pointing up and press dose button until 0 mg lines up with pointer (See Figure G). Repeat steps C and D, up to 6 times, until a drop of Victoza appears at the needle tip.
- Take your Victoza pen from where it is stored.
- Wash hands with soap and water before use.
- Check pen label before each use to make sure it is your Victoza pen.
- Pull off pen cap (See Figure H).
- Check Victoza in the cartridge. The liquid should be clear, colorless and free of particles. If not, do not use.
- Wipe the rubber stopper with an alcohol swab.
- Remove protective tab from outer needle cap.
- Push outer needle cap containing the needle straight onto the pen, then screw needle on until secure (See Figure I).
- Pull off outer needle cap. Do not throw away (See Figure J).
- Pull off inner needle cap and throw away (See Figure K). A small drop of liquid may appear. This is normal.
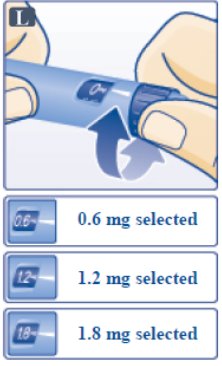
- Victoza pen can give a dose of 0.6 mg (starting dose), 1.2 mg or 1.8 mg. Be sure that you know the dose of Victoza that is prescribed for you.
- Turn the dose selector until your needed dose lines up with the pointer (0.6 mg, 1.2 mg or 1.8 mg) (See Figure L).
- You will hear a "click" every time you turn the dose selector.
- If you select a wrong dose, change it by turning the dose selector backwards or forwards until the correct dose lines up with the pointer. Be careful not to press the dose button when turning the dose selector. This may cause Victoza to come out.
- Insert needle into your skin in the stomach (abdomen), thigh or upper arm. Use the injection technique shown to you by your healthcare provider.
- Press down on the center of the dose button to inject until 0 mg lines up with the pointer (See Figure M).
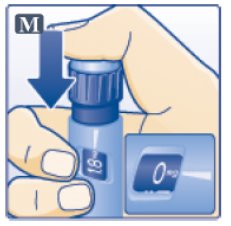
- Be careful not to touch the dose display with your other fingers. This may block the injection.
- Keep the dose button pressed down and make sure that you keep the needle under the skin for a full count of 6 seconds to make sure the full dose is injected. Keep your thumb on the injection button until you remove the needle from your skin (See Figure N).
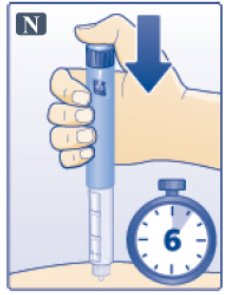
- Change (rotate) your injection sites within the area you choose for each dose.
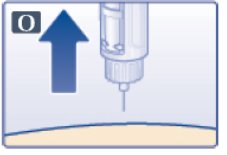
- You may see a drop of Victoza at the needle tip. This is normal and it does not affect the dose you just received. If blood appears after you take the needle out of your skin, apply light pressure, but
- Carefully put the outer needle cap over the needle (See Figure P). Unscrew the needle.
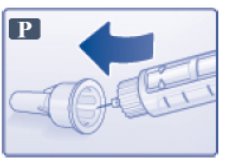
- Safely remove the needle from your Victoza pen after each use.
- Put your used VICTOZA pen and needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and pens in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles with other people. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- After removing the needle, put the pen cap on your Victoza pen and store your Victoza pen without the needle attached (See Figure Q).
- Do not try to refill your Victoza pen - it is prefilled and is disposable.
- Do not try to repair your pen or pull it apart.
- Keep your Victoza pen away from dust, dirt and liquids.
- If cleaning is needed, wipe the outside of the pen with a clean, damp cloth.
- Store your new, unused Victoza pen in the refrigerator at 36ºF to 46ºF (2ºC to 8ºC).
- If Victoza is stored outside of refrigeration (by mistake) prior to first use, it should be used or thrown away within 30 days.
- Do not freeze Victoza or use Victoza if it has been frozen. Do not store Victoza near the refrigerator cooling element.
- Use a Victoza pen for only 30 days. Throw away a used Victoza pen 30 days after you start using it, even if some medicine is left in the pen.
- Store your Victoza pen at 59ºF to 86ºF (15ºC to 30ºC), or in a refrigerator at 36ºF to 46ºF (2°C to 8°C).
- When carrying the pen away from home, store the pen at a temperature between 59ºF to 86ºF (15ºC to 30ºC).
- If Victoza has been exposed to temperatures above 86ºF (30°C), it should be thrown away.
- Protect your Victoza pen from heat and sunlight.
- Keep the pen cap on when your Victoza pen is not in use.
- Always remove the needle after each injection and store your pen without the needle attached. This reduces the risk of contamination, infection, leakage and inaccurate dosing.
