Generic Name
Mirtazapine
Brand Names
Remeron, Remeronsoltab
FDA approval date: June 19, 2003
Form: Tablet
What is Remeron (Mirtazapine)?
REMERON/REMERONSolTab are indicated for the treatment of major depressive disorder in adults. REMERON/REMERONSolTab is indicated for the treatment of major depressive disorder in adults.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
REMERON (MIRTAZAPINE)
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors
1INDICATIONS AND USAGE
REMERON/REMERONSolTab are indicated for the treatment of major depressive disorder (MDD) in adults
2DOSAGE FORMS AND STRENGTHS
REMERON is supplied as:
- 15 mg tablets: Oval, scored, yellow, with "MSD" debossed on one side and "
- 30 mg tablets: Oval, scored, red-brown, with "MSD" debossed on one side and "
REMERONSolTab is supplied as:
- 15 mg orally disintegrating tablets: Round, white, with "
- 30 mg orally disintegrating tablets: Round, white, with "
- 45 mg orally disintegrating tablets: Round, white, with "
3CONTRAINDICATIONS
REMERON/REMERONSolTab is contraindicated in patients:
- Taking, or within 14 days of stopping, MAOIs (including the MAOIs linezolid and intravenous methylene blue) because of an increased risk of serotonin syndrome
- With a known hypersensitivity to mirtazapine or to any of the excipients in REMERON/REMERONSolTab
4ADVERSE REACTIONS
The following adverse reactions are described in more detail in other sections of the prescribing information:
- Hypersensitivity
- Suicidal Thoughts and Behaviors
- Agranulocytosis
- Serotonin Syndrome
- Angle-Closure Glaucoma
- QT Prolongation and Torsades de Pointes
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
- Increased Appetite and Weight Gain
- Somnolence
- Activation of Mania or Hypomania
- Seizures
- Elevated Cholesterol and Triglycerides
- Hyponatremia
- Transaminase Elevations
- Discontinuation Syndrome
- Use in Patients with Concomitant Illness
4.1Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below are from clinical trials in which REMERON/REMERONSolTab was administered to 2796 patients in phase 2 and 3 clinical studies. The trials consisted of double-blind controlled and open-label studies, inpatient and outpatient studies, fixed dose, and titration studies.
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of REMERON. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac disorders: ventricular arrhythmia (Torsades de Pointes)
Endocrine disorders: hyperprolactinemia (and related symptoms, e.g., galactorrhea and gynecomastia)
Musculoskeletal and connective tissue disorders: increased creatine kinase blood levels and rhabdomyolysis
Psychiatric disorders: somnambulism (ambulation and other complex behaviors out of bed)
Reproductive system and breast disorders: priapism
Skin and subcutaneous tissue disorders: severe skin reactions, including DRESS, Stevens-Johnson syndrome, bullous dermatitis, erythema multiforme and toxic epidermal necrolysis
5DRUG INTERACTIONS
Table 5 includes clinically important drug interactions with REMERON/REMERONSolTab
6DESCRIPTION
REMERON and REMERONSolTab contain mirtazapine. Mirtazapine has a tetracyclic chemical structure and belongs to the piperazino-azepine group of compounds. It is designated 1,2,3,4,10,14b-hexahydro-2-methylpyrazino [2,1-a] pyrido [2,3-c][2] benzazepine and has the empirical formula of C
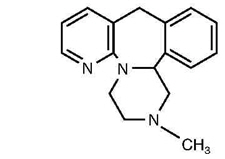
Mirtazapine is a white to creamy white crystalline powder which is practically insoluble in water.
REMERON is available for oral administration as scored film-coated tablets containing 15 or 30 mg of mirtazapine Each tablet contains the following inactive ingredients: colloidal silicon dioxide anhydrous, corn starch, ferric oxide (yellow), hydroxypropyl cellulose, hypromellose, magnesium stearate, lactose monohydrate, polyethylene glycol 8000, and titanium dioxide. The 30 mg tablets also contain ferric oxide (red).
REMERONSolTab is available for oral administration as an orally disintegrating tablet containing 15, 30, or 45 mg of mirtazapine. REMERONSolTab also contains the following inactive ingredients: aspartame, citric acid anhydrous fine granular, crospovidone, hypromellose, magnesium stearate, mannitol, granular mannitol 2080, microcrystalline cellulose, natural and artificial orange flavor, polymethacrylate (Eudragit E100), povidone, sodium bicarbonate, and sugar spheres (composed of starch and sucrose).
7CLINICAL STUDIES
The efficacy of REMERON as a treatment for major depressive disorder was established in 4 placebo-controlled, 6-week trials in adult outpatients meeting DSM-III criteria for major depressive disorder. Patients were titrated with REMERON from a dose range of 5 mg to 35 mg/day. The mean mirtazapine dose for patients who completed these 4 studies ranged from 21 to 32 mg/day. Overall, these studies demonstrated REMERON to be superior to placebo on at least 3 of the following 4 measures: 21-Item Hamilton Depression Rating Scale (HDRS) total score; HDRS Depressed Mood Item; CGI Severity score; and Montgomery and Asberg Depression Rating Scale (MADRS). Superiority of REMERON over placebo was also found for certain factors of the HDRS, including anxiety/somatization factor and sleep disturbance factor.
Examination of age and gender subsets of the population did not reveal any differential responsiveness on the basis of these subgroupings.
In a longer-term study, patients meeting (DSM-IV) criteria for major depressive disorder who had responded during an initial 8 to 12 weeks of acute treatment on REMERON were randomized to continuation of REMERON or placebo for up to 40 weeks of observation for relapse. Response during the open phase was defined as having achieved a HAM-D 17 total score of ≤8 and a CGI-Improvement score of 1 or 2 at 2 consecutive visits beginning with week 6 of the 8 to 12 weeks in the open-label phase of the study. Relapse during the double-blind phase was determined by the individual investigators. Patients receiving continued REMERON treatment experienced significantly lower relapse rates over the subsequent 40 weeks compared to those receiving placebo. This pattern was demonstrated in both male and female patients.
8HOW SUPPLIED/STORAGE AND HANDLING
REMERON tablets are supplied as:
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
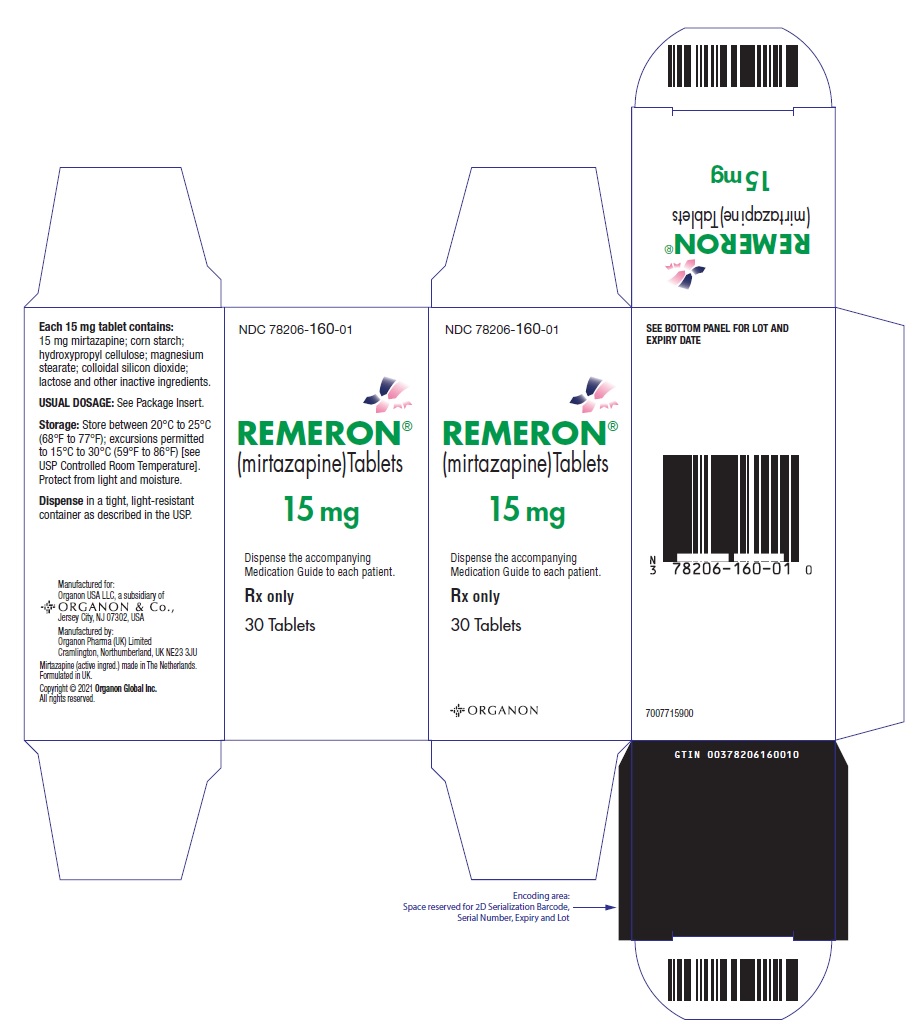
10PRINCIPAL DISPLAY PANEL - 15 mg Tablet Bottle Label
NDC 78206-160-01
REMERON®
(mirtazapine)Tablets
(mirtazapine)Tablets
15 mg
Rx only
30 Tablets
Must be dispensed with Medication Guide.
Manuf. for: Organon USA LLC, a subsidiary of
ORGANON & Co.,
Jersey City, NJ 07302, USA
Manuf. by: Organon Pharma (UK) Limited
Cramlington, Northumberland, UK NE23 3JU
Mirtazapine (active ingred.) made in The Netherlands.
Formulated in UK.
Manuf. for: Organon USA LLC, a subsidiary of
ORGANON & Co.,
Jersey City, NJ 07302, USA
Manuf. by: Organon Pharma (UK) Limited
Cramlington, Northumberland, UK NE23 3JU
Mirtazapine (active ingred.) made in The Netherlands.
Formulated in UK.
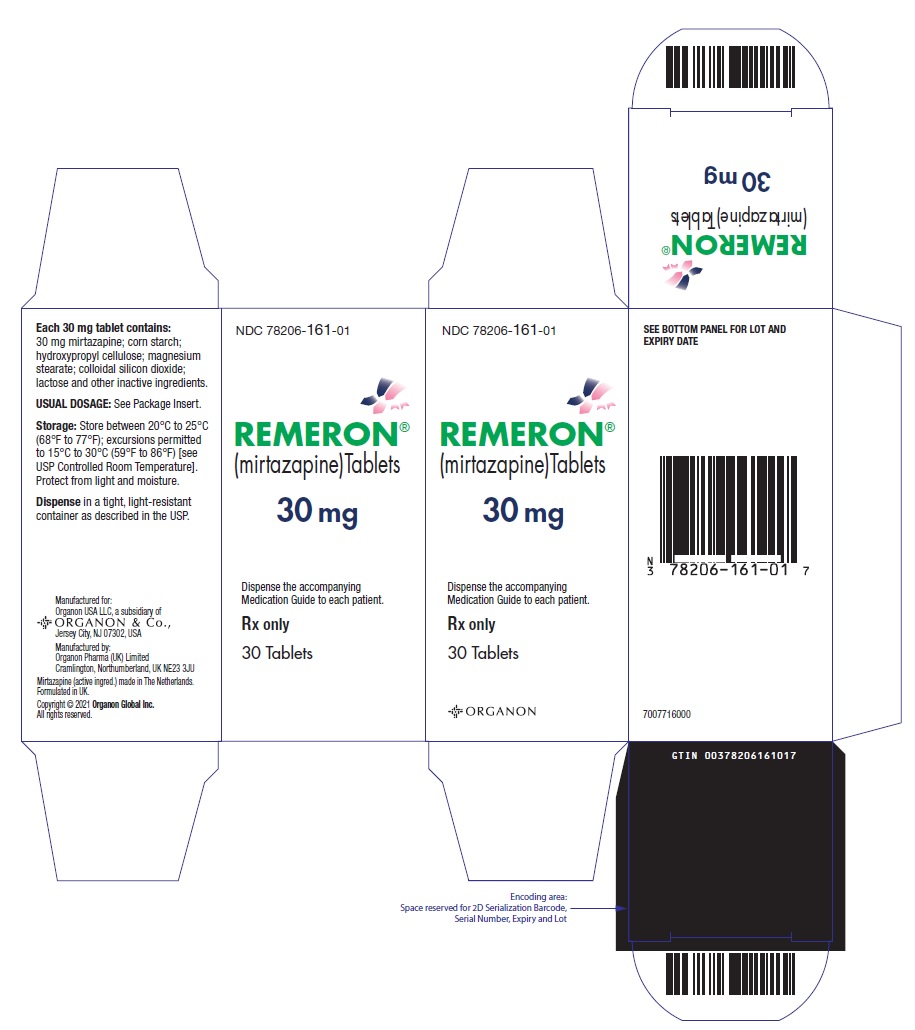
11PRINCIPAL DISPLAY PANEL - 30 mg Tablet Bottle Label
NDC 78206-161-01
REMERON®
(mirtazapine)Tablets
(mirtazapine)Tablets
30 mg
Rx only
30 Tablets
Must be dispensed with Medication Guide.
Manuf. for: Organon USA LLC, a subsidiary of
ORGANON & Co.,
Jersey City, NJ 07302, USA
Manuf. by: Organon Pharma (UK) Limited
Cramlington, Northumberland, UK NE23 3JU
Mirtazapine (active ingred.) made in The Netherlands.
Formulated in UK.
Manuf. for: Organon USA LLC, a subsidiary of
ORGANON & Co.,
Jersey City, NJ 07302, USA
Manuf. by: Organon Pharma (UK) Limited
Cramlington, Northumberland, UK NE23 3JU
Mirtazapine (active ingred.) made in The Netherlands.
Formulated in UK.
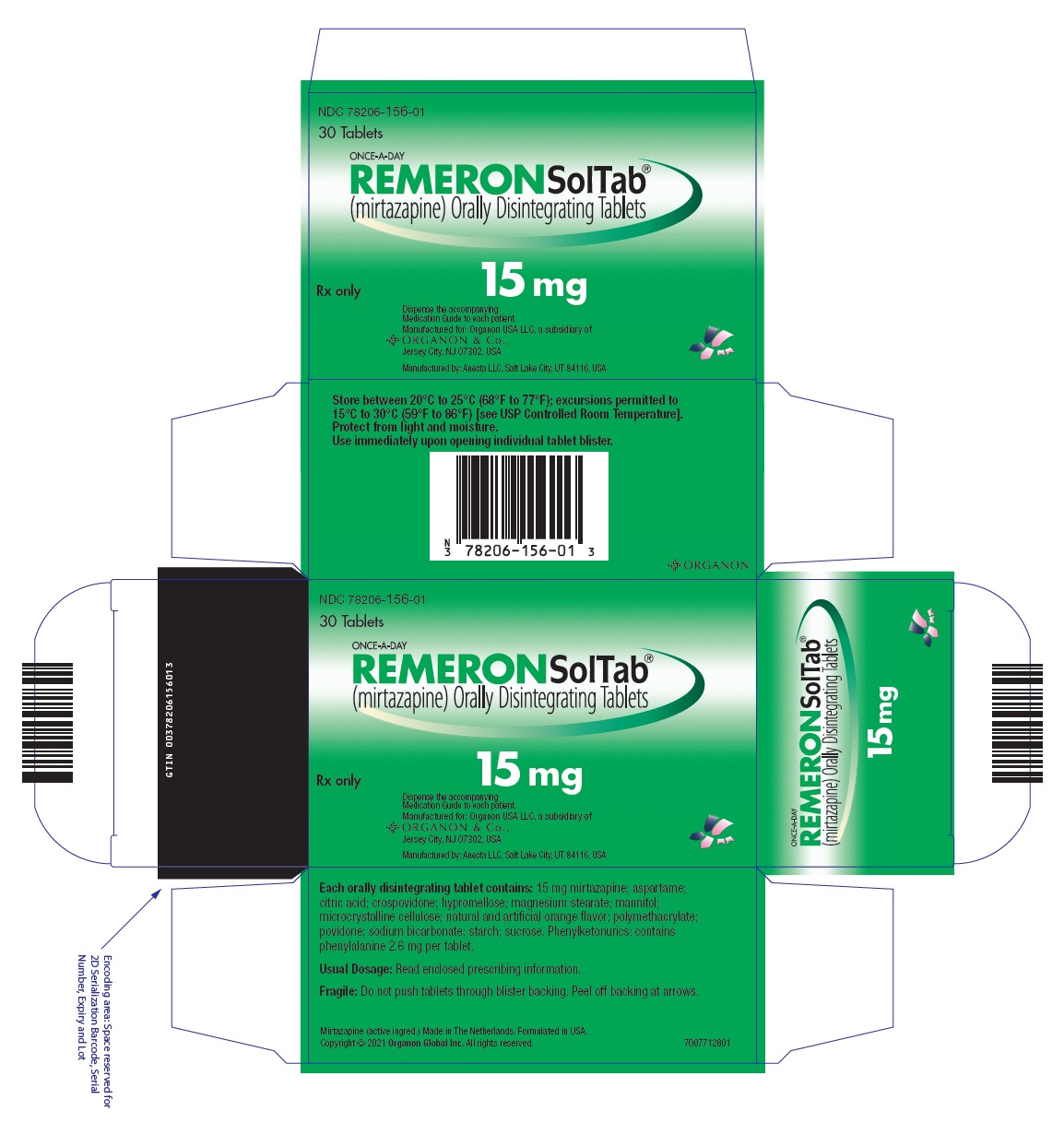
12PRINCIPAL DISPLAY PANEL - 15 mg Tablet Blister Pack Box
NDC 78206-156-01
30 Tablets
ONCE-A-DAY
Rx only
15 mg
Dispense the accompanying
Manufactured by: Anesta LLC, Salt Lake City, UT 84116, USA
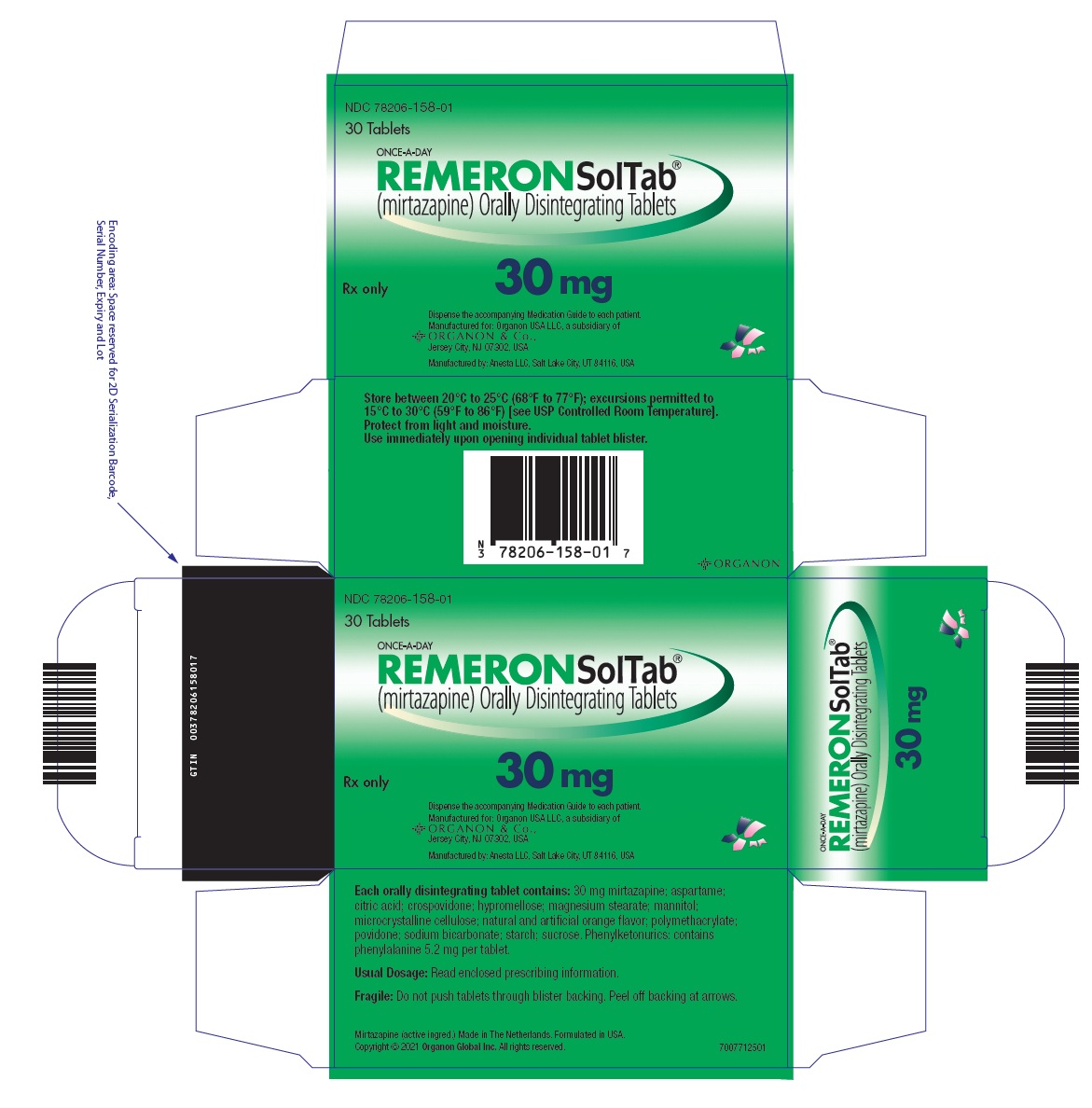
13PRINCIPAL DISPLAY PANEL - 30 mg Tablet Blister Pack Box
NDC 78206-158-01
30 Tablets
ONCE-A-DAY
Rx only
30 mg
Dispense the accompanying Medication Guide to each patient.
Manufactured by: Anesta LLC, Salt Lake City, UT 84116, USA
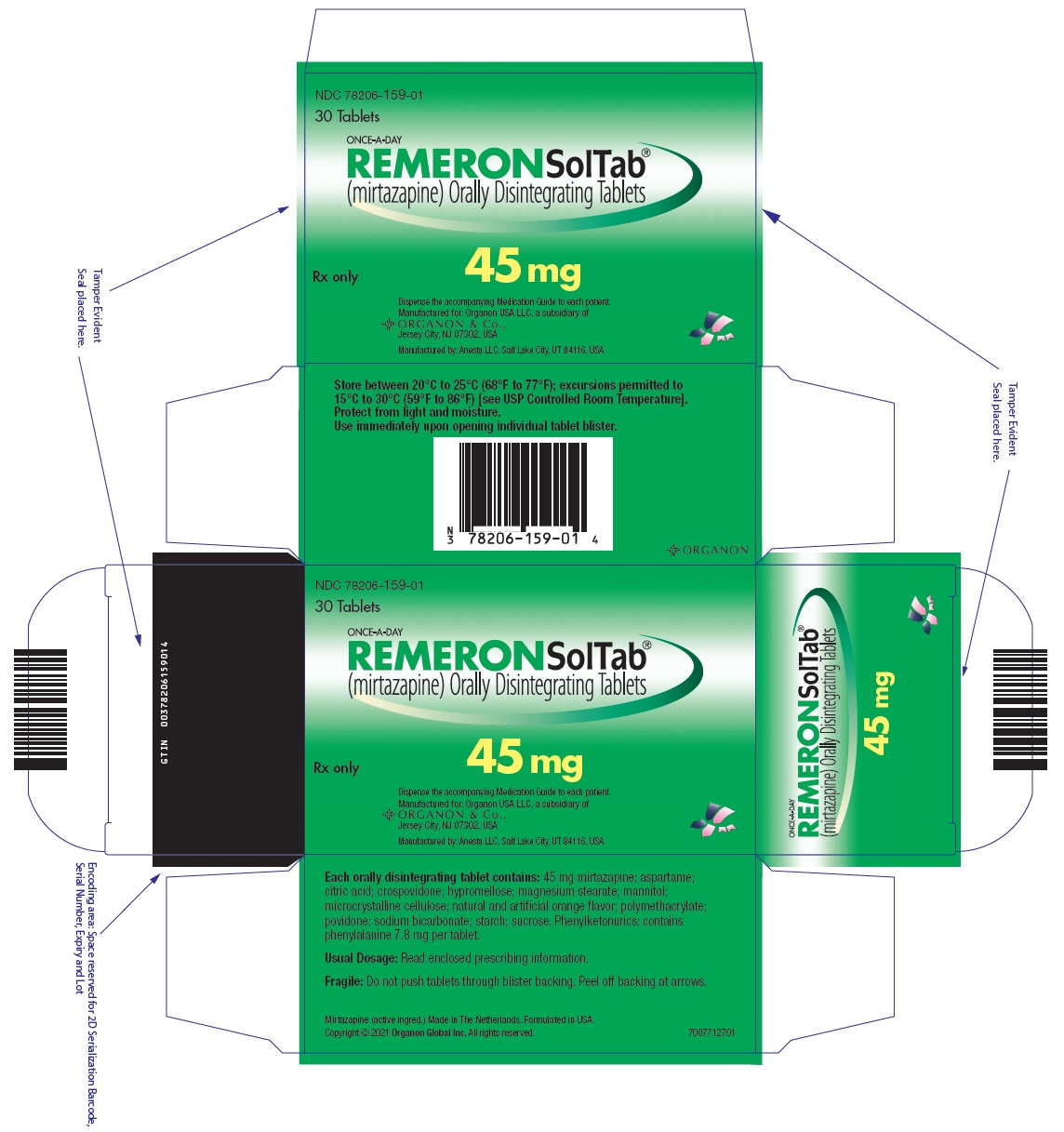
14PRINCIPAL DISPLAY PANEL - 45 mg Tablet Blister Pack Box
NDC 78206-159-01
30 Tablets
ONCE-A-DAY
Rx only
45 mg
Dispense the accompanying Medication Guide to each patient.
Manufactured by: Anesta LLC, Salt Lake City, UT 84116, USA