Brand Name
Vizamyl
Generic Name
Flutemetamol F-18
View Brand Information FDA approval date: January 01, 2014
Classification: Radioactive Diagnostic Agent
Form: Solution
What is Vizamyl (Flutemetamol F-18)?
Vizamyl is indicated for Positron Emission Tomography imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer's Disease and other causes of cognitive decline. A negative Vizamyl scan indicates sparse to no neuritic plaques and is inconsistent with a neuropathological diagnosis of AD at the time of image acquisition; a negative scan result reduces the likelihood that a patient's cognitive impairment is due to AD. A positive Vizamyl scan indicates moderate to frequent amyloid neuritic plaques; neuropathological examination has shown this amount of amyloid neuritic plaque is present in patients with AD, but may also be present in patients with other types of neurologic conditions as well as in older people with normal cognition. Vizamyl is an adjunct to other diagnostic evaluations. Limitations of Use: A positive Vizamyl scan does not establish a diagnosis of AD or other cognitive disorder. Safety and effectiveness of Vizamyl have not been established for: Predicting development of dementia or other neurologic condition. Monitoring responses to therapies. Vizamyl is a radioactive diagnostic agent indicated for Positron Emission Tomography imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer's disease or other causes of cognitive decline. A negative Vizamyl scan indicates sparse to no neuritic plaques, and is inconsistent with a neuropathological diagnosis of AD at the time of image acquisition; a negative scan result reduces the likelihood that a patient's cognitive impairment is due to AD. A positive Vizamyl scan indicates moderate to frequent amyloid neuritic plaques; neuropathological examination has shown this amount of neuritic plaque is present in patients with AD, but may also be present in patients with other types of neurologic conditions, as well as older people with normal cognition. Vizamyl is an adjunct to other diagnostic evaluations . Limitations of Use: A positive Vizamyl scan does not establish a diagnosis of AD or other cognitive disorder Safety and effectiveness of Vizamyl have not been established for: Predicting development of dementia or other neurological condition Monitoring responses to therapies
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Vizamyl (Flutemetamol F-18)
1INDICATIONS AND USAGE
VIZAMYL is indicated for positron emission tomography (PET) of the brain to estimate amyloid beta neuritic plaque density in adults with cognitive impairment for:
- Evaluation of Alzheimer's disease (AD) and other causes of cognitive decline
- Selection of patients who are indicated for amyloid beta-directed therapy as described in the prescribing information of the therapeutic products
2DOSAGE FORMS AND STRENGTHS
Injection: 150 MBq/mL (4.05 mCi/mL) of flutemetamol F 18 in up to 30 mL volume at reference date and time as a clear, colorless to slightly yellow solution in a multiple-dose vial.
3CONTRAINDICATIONS
VIZAMYL is contraindicated in patients with a history of hypersensitivity reaction to VIZAMYL or polysorbate 80
4ADVERSE REACTIONS
The following clinically significant adverse reaction is described elsewhere in the labeling:
- Hypersensitivity Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of VIZAMYL was evaluated in 761 adult subjects who received VIZAMYL by intravenous injection in clinical trials. Most subjects (70%) received a dose of 185 MBq (5 mCi). The subjects had a mean age of 62 years (range 18 years to 93 years); 45% of the subjects were male and 91% were White.
A serious hypersensitivity reaction characterized by flushing, dyspnea, and chest pressure was reported within minutes following VIZAMYL administration in one subject who recovered with treatment.
Adverse reactions reported in ≥ 1% of subjects from the clinical trials are shown in Table 2.
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of VIZAMYL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
5OVERDOSAGE
The major risks of overdose relate predominantly to increased radiation exposure, with long-term risks for neoplasia. In the event of administration of a radiation overdose with VIZAMYL, hydration and frequent urination should be encouraged to minimize radiation exposure to the subject. It is unknown whether or not flutemetamol is dialyzable.
6PRINCIPAL DISPLAY PANEL - 30 mL Vial Label
NDC 17156-067-30
Multiple-Dose Vial
CAUTION
Vizamyl™
Batch No.: ___________________ Date: ______________________Vial No.:_______________
Distributed by GE HealthCare, Medi-Physics Inc., Arlington Heights, IL 60004, U.S.A.
40-3067A
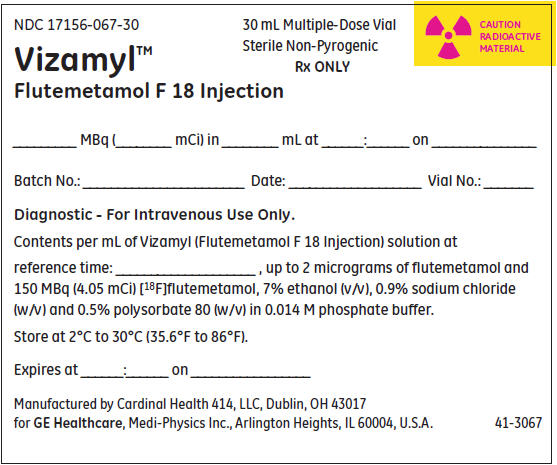
7PRINCIPAL DISPLAY PANEL - 30 mL Vial Container Label
NDC 17156-067-30
Multiple-Dose Vial
CAUTION
Vizamyl™
_______ MBq (________ mCi) in ________ mL at ______:______on _______________
Batch No.: _______________________ Date: ___________________ Vial No.: _______
Diagnostic - For Intravenous Use Only.
Recommended dosage: See prescribing information.
Contents per mL of Vizamyl (flutemetamol F 18 injection) solution at
Expires at ______:______ on _________________
Distributed by GE HealthCare, Medi-Physics Inc., Arlington Heights, IL 60004, U.S.A.
41-3067A

