Takhzyro
What is Takhzyro (Lanadelumab-Flyo)?
For people living with hereditary angioedema (HAE), life can be unpredictable and frightening. Sudden swelling attacks often affecting the face, hands, feet, throat, or abdomen can appear without warning and, in severe cases, become life-threatening. Managing this rare condition requires more than reacting to attacks; it requires preventing them before they start. Takhzyro (lanadelumab-flyo) was developed to do exactly that, helping patients gain control, stability, and confidence in their daily lives.
Takhzyro is a prescription injectable medication used to help prevent attacks of hereditary angioedema in adults and children aged 2 years and older. It belongs to a class of drugs known as plasma kallikrein inhibitors. Approved by the U.S. Food and Drug Administration (FDA) in 2018, Takhzyro is considered a preventive (prophylactic) therapy for HAE. Unlike medications that treat acute swelling episodes, it works continuously to reduce their frequency, often leading to dramatic improvements in quality of life.
What does Takhzyro do?
Takhzyro helps prevent swelling attacks in people with hereditary angioedema (HAE), a rare genetic disorder caused by a deficiency or dysfunction of a protein called C1 esterase inhibitor. Without enough of this protein, the body produces too much of an enzyme called plasma kallikrein, which leads to excessive bradykinin, a chemical that causes blood vessels to leak fluid into surrounding tissues. This leakage triggers the sudden and painful swelling characteristic of HAE.
By inhibiting plasma kallikrein, Takhzyro helps reduce or prevent these swelling episodes from occurring in the first place. Patients who use Takhzyro often report fewer HAE attacks, less severe symptoms, and longer periods of normal, symptom-free life.
In clinical trials, many patients experienced a 70–90% reduction in attack frequency, and some became completely attack-free after several months of treatment (FDA, 2024). For individuals who once lived in constant fear of an unpredictable attack, this can be life-changing.
How does Takhzyro work?
Takhzyro contains lanadelumab-flyo, a fully human monoclonal antibody that targets and blocks the activity of plasma kallikrein, the key enzyme involved in producing bradykinin.
When plasma kallikrein levels rise uncontrollably, bradykinin increases, and blood vessels become leaky, leading to swelling. By binding to plasma kallikrein and preventing its activity, Takhzyro reduces the formation of bradykinin, keeping the blood vessels stable and preventing swelling episodes.
This mechanism provides long-term protection against attacks rather than temporary relief. Over time, patients often notice fewer flare-ups, reduced anxiety about future episodes, and more freedom to engage in work, travel, and social activities.
Clinically, this targeted approach matters because it directly addresses the underlying biochemical imbalance in HAE, rather than just treating symptoms once they appear.
Takhzyro side effects
Most patients tolerate Takhzyro well, but side effects can occur, as with any medication. Fortunately, most are mild to moderate and manageable with medical guidance.
Common side effects may include:
- Injection site reactions (pain, redness, swelling, or itching)
- Headache or dizziness
- Fatigue or mild muscle pain
Less common but potentially serious side effects:
- Allergic reactions such as rash, hives, or swelling of the face or throat
- Difficulty breathing
- Signs of infection (fever, chills, or persistent sore throat)
If any severe or unusual symptoms occur, patients should seek immediate medical attention.
Takhzyro should be used with caution in individuals who have had allergic reactions to lanadelumab or other components of the formulation. It is not known to cause significant liver or kidney toxicity, but all patients should follow their healthcare provider’s instructions closely and attend recommended follow-up appointments.
Importantly, Takhzyro is not used to treat an ongoing HAE attack. Patients must continue to keep an “on-demand” treatment available for emergencies, as advised by their doctor.
Takhzyro dosage
Takhzyro is a preventative subcutaneous injection given in the abdomen, thigh, or upper arm. It can be administered by a healthcare professional or self-administered after proper training. Dosage frequency varies, so patients should follow their provider’s schedule.
Doctors may monitor attack frequency, symptom patterns, and any side effects to adjust treatment if necessary. Routine lab testing is generally not required, as Takhzyro does not typically affect liver or kidney function.
For children, older adults, or individuals with other medical conditions, dosing recommendations may differ slightly, but clinical studies have shown that Takhzyro is safe and effective across age groups when used as prescribed.
Does Takhzyro have a generic version?
As of 2025, Takhzyro (lanadelumab-flyo) does not have an FDA-approved generic version available in the United States or internationally. It is currently marketed exclusively by Takeda Pharmaceuticals, which developed the drug as part of its commitment to rare disease treatment. However, international versions may exist in other markets.
Takhzyro, a biologic medication, will eventually have a biosimilar, a nearly identical drug requiring separate FDA approval. Patients can explore financial assistance or insurance options from Takeda and advocacy groups until a biosimilar is available.
Conclusion
Takhzyro (lanadelumab-flyo) represents a significant advancement in the prevention of hereditary angioedema attacks. By targeting plasma kallikrein and reducing bradykinin levels, it helps patients experience fewer and sometimes no swelling episodes.
For many, this means freedom from the constant fear of unpredictable attacks and a chance to live life on their own terms. While side effects can occur, they are generally mild and manageable under medical supervision.
Every patient’s journey with HAE is unique, but with Takhzyro, there is now a proven option that offers long-term protection, improved quality of life, and renewed peace of mind.
References
- U.S. Food and Drug Administration (FDA). (2024). Takhzyro (lanadelumab-flyo) prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Lanadelumab injection: Uses, dosage, and side effects. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Lanadelumab-flyo injection information. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Hereditary angioedema: Treatment and management updates. Retrieved from https://www.nih.gov
Approved To Treat
Related Clinical Trials
Summary: This study is a survey in Japan of Lanadelumab used to treat people with hereditary angioedema (HAE). The study sponsor will not be involved in how the participants are treated but will provide instructions on how the clinics will record what happens during the study. The main aim of the study is to check for side effects related from Lanadelumab and to check if Lanadelumab improves symptoms of HA...
Related Latest Advances
Brand Information
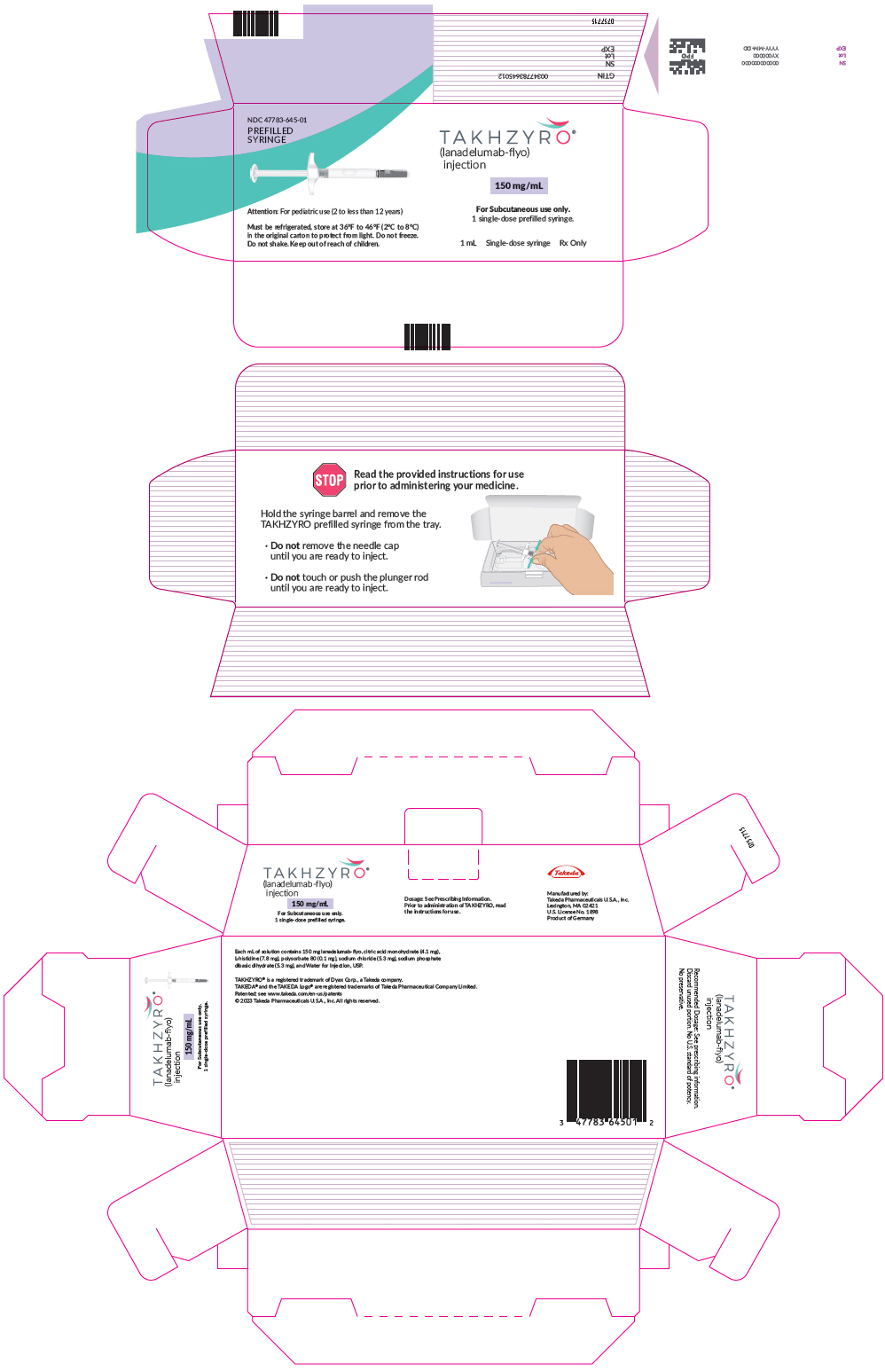
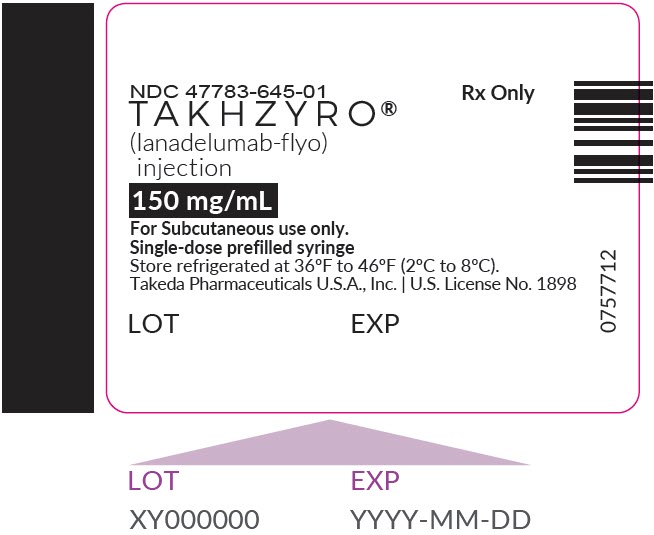
- Injection: 150 mg/1 mL (150 mg/mL) solution in a single-dose prefilled syringe
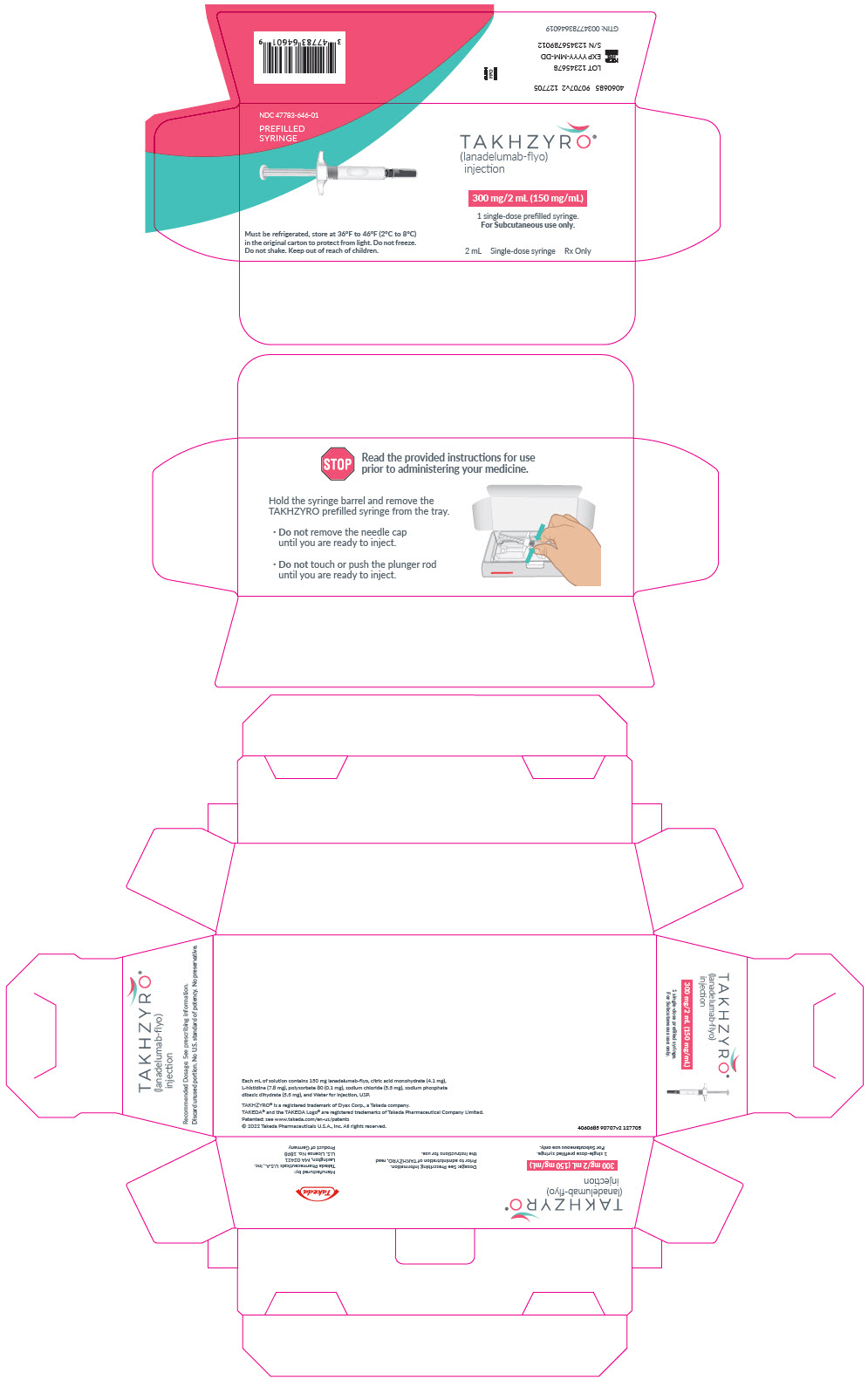
- Injection: 300 mg/2 mL (150 mg/mL) solution in a single-dose prefilled syringe
- Injection: 300 mg/2 mL (150 mg/mL) solution in a single-dose vial
- TAKHZYRO is a ready-to-use solution for injection under the skin (subcutaneous). It is supplied in a single-dose, glass vial.
- Your healthcare provider will prescribe the dose that you should take.
- Only use the syringes, transfer needles, and injection needles that your healthcare provider prescribes.
- Only use the syringes, transfer needles and injection needles 1 time. Throw away (dispose of) any used syringes and needles.
- Store TAKHZYRO in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Store TAKHZYRO in the original carton to protect the vial from light.
- Do not shake TAKHZYRO.
- Keep TAKHZYRO and all medicines out of the reach of children.
- Gather all supplies and place them on a well-lighted flat work surface.
- Take the vial out of the refrigerator 15 minutes before use and allow it to reach room temperature before preparing an injection.
- Check the expiration date on the box and vial label of TAKHZYRO.
- Check the supplies for damage.
- Clean your work area and wash your hands before preparing your dose.
- Remove the vial from the packaging.
(lanadelumab-flyo)
injection
(lanadelumab-flyo)
injection

(lanadelumab-flyo)
injection
1 single-dose prefilled syringe.