Brand Name
Exelderm
Generic Name
Sulconazole
View Brand Information FDA approval date: June 07, 2019
Classification: Azole Antifungal
Form: Cream, Solution
What is Exelderm (Sulconazole)?
EXELDERM SOLUTION, 1.0% is a broad-spectrum antifungal agent indicated for the treatment of tinea cruris and tinea corporis caused by Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis; and for the treatment of tinea versicolor. Effectiveness has not been proven in tinea pedis . Symptomatic relief usually occurs within a few days after starting EXELDERM SOLUTION and clinical improvement usually occurs within one week.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Exelderm (Sulconazole Nitrate)
1DESCRIPTION
EXELDERM (sulconazole nitrate, USP) CREAM, 1.0% is a broad-spectrum antifungal agent intended for topical application. Sulconazole nitrate, USP, the active ingredient in EXELDERM CREAM, is an imidazole derivative with
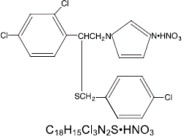
Sulconazole nitrate, USP is a white to off-white crystalline powder with a molecular weight of 460.77. It is freely soluble in pyridine; slightly soluble in ethanol, acetone, and chloroform; and very slightly soluble in water. It has a melting point of about 130°C.
EXELDERM CREAM contains sulconazole nitrate, USP 10 mg/g in an emollient cream base consisting of propylene glycol, stearyl alcohol, isopropyl myristate, cetyl alcohol, polysorbate 60, sorbitan mono-stearate, glyceryl stearate (and) PEG-100 stearate, ascorbyl paImitate, and purified water, with sodium hydroxide and/or nitric acid added to adjust the pH.
2CLINICAL PHARMACOLOGY
Sulconazole nitrate is an imidazole derivative with broad-spectrum antifungal activity that inhibits the growth
A modified Draize test showed no allergic contact dermatitis and a phototoxicity study showed no phototoxic or photoallergic reaction to sulconazole nitrate cream. Maximization tests with sulconazole nitrate cream showed no evidence of contact sensitization or irritation.
3INDICATIONS AND USAGE
EXELDERM (sulconazole nitrate, USP) CREAM, 1.0% is an antifungal agent indicated for the treatment of tinea pedis (athlete's foot), tinea cruris, and tinea corporis caused by
*Efficacy for this organism in the organ system was studied in fewer than ten infections.
4CONTRAINDICATIONS
EXELDERM (sulconazole nitrate, USP) CREAM, 1.0% is contraindicated in patients who have a history of hypersensitivity to any of its ingredients.
5ADVERSE REACTIONS
There were no systemic effects and only infrequent cutaneous adverse reactions in 1185 patients treated with sulconazole nitrate cream in controlled clinical trials. Approximately 3% of these patients reported itching, 3% burning or stinging, and 1% redness. These complaints did not usually interfere with treatment.
6CLINICAL STUDIES
In a vehicle-controlled study for the treatment of tinea pedis (moccasin type) due to
7DOSAGE AND ADMINISTRATION
A small amount of cream should be gently massaged into the affected and surrounding skin areas once or twice daily, except in tinea pedis, where administration should be twice daily.
Early relief of symptoms is experienced by the majority of patients and clinical improvement may be seen fairly soon after treatment is begun; however, tinea corporis/cruris and tinea versicolor should be treated for 3 weeks and tinea pedis for 4 weeks to reduce the possibility of recurrence.
If significant clinical improvement is not seen after 4 to 6 weeks of treatment, an alternate diagnosis should be considered.
8HOW SUPPLIED
EXELDERM (sulconazole nitrate, USP) CREAM, 1.0% is a smooth, glossy white to off-white cream having a slight characteristic odor. It is supplied as follows:
60 g tube – NDC 69489-711-60
Avoid excessive heat, above 40° C (104° F).
To report SUSPECTED ADVERSE REACTIONS, contact the FDA at 1-800-FDA-1088 or
JOURNEY
Manufactured for:
141113
9Principal Display Panel – 60 g Carton Label
Exelderm®
(sulconazole nitrate, USP)
NDC 69489-711-60
Net Wt. 60 g
JOURNEY
MEDICAL CORPORATION
10Principal Display Panel – 60 gram Tube Label
Exelderm®
(sulconazole nitrate, USP)
Avoid excessive heat, above 40°C (104° F).
NDC 69489-711-60
Rx only
Net Wt. 60 g
For topical use only.
Not for ophthalmic use.
