Generic Name
Solifenacin
Brand Names
VESIcare, Solifenacine
FDA approval date: January 05, 2005
Classification: Cholinergic Muscarinic Antagonist
Form: Tablet, Suspension
What is VESIcare (Solifenacin)?
Solifenacin succinate tablets are indicated for the treatment of adults with overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency. Solifenacin succinate tablets are a muscarinic antagonist indicated for the treatment of adults with overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
VESIcare (solifenacin succinate)
1INDICATIONS AND USAGE
VESIcare
2DOSAGE FORMS AND STRENGTHS
Tablets:
- 5 mg: round, light yellow, debossed with 150
- 10 mg: round, light pink, debossed with 151
3CONTRAINDICATIONS
VESIcare is contraindicated in patients:
- With urinary retention
- With gastric retention
- With uncontrolled narrow-angle glaucoma
- Who have demonstrated hypersensitivity to solifenacin succinate or the inactive ingredients in VESIcare. Reported adverse reactions have included anaphylaxis and angioedema
4OVERDOSAGE
Overdosage with VESIcare can potentially result in severe antimuscarinic effects and should be treated accordingly. The highest dose ingested in an accidental overdose of solifenacin succinate was 280 mg (28 times the maximum dosage) in a 5-hour period. This case was associated with mental status changes. Some cases reported a decrease in the level of consciousness.
Intolerable antimuscarinic adverse reactions (fixed and dilated pupils, blurred vision, failure of heel-to-toe exam, tremors, and dry skin) occurred on day 3 in normal volunteers taking 50 mg daily (5 times the maximum recommended therapeutic dose) and resolved within 7 days following discontinuation of drug.
In the event of overdose with VESIcare, treat with gastric lavage and appropriate supportive measures. ECG monitoring is also recommended.
5DESCRIPTION
VESIcare (solifenacin succinate) is a muscarinic receptor antagonist. Chemically, solifenacin succinate is a butanedioic acid compound with (1
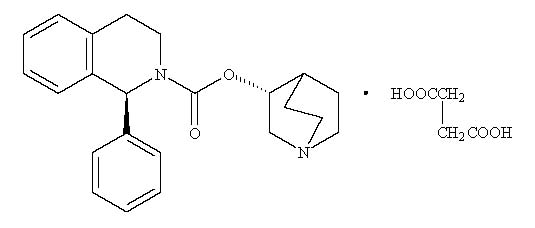
Solifenacin succinate is a white to pale-yellowish-white crystal or crystalline powder. It is freely soluble at room temperature in water, glacial acetic acid, dimethyl sulfoxide, and methanol.
Each VESIcare tablet contains 5 or 10 mg of solifenacin succinate and is for oral administration. In addition to the active ingredient solifenacin succinate, each VESIcare tablet also contains the following inactive ingredients: lactose monohydrate, corn starch, hypromellose 2910, magnesium stearate, talc, polyethylene glycol 8000 and titanium dioxide with yellow ferric oxide (5 mg VESIcare tablet) or red ferric oxide (10 mg VESIcare tablet).
6CLINICAL STUDIES
VESIcare was evaluated in four twelve-week, double-blind, randomized, placebo-controlled, parallel group, multicenter clinical trials for the treatment of overactive bladder in adult patients having symptoms of urinary frequency, urgency, and/or urge or mixed incontinence (with a predominance of urge). Entry criteria required that patients have symptoms of overactive bladder for ≥ 3 months duration. These studies involved 3027 patients (1811 on VESIcare and 1216 on placebo), and approximately 90% of these patients completed the 12-week studies. Two of the four studies evaluated the 5 and 10 mg VESIcare doses (Studies 1 and 2) and the other two evaluated only the 10 mg dose (Studies 3 and 4). All patients completing the 12-week studies were eligible to enter an open-label, long-term extension study (Study 5) and 81% of patients enrolling completed the additional 40-week treatment period. The majority of patients were Caucasian (93%) and female (80%) with a mean age of 58 years.
The primary endpoint in all four trials was the mean change from baseline to 12 weeks in number of micturitions/24 hours. Secondary endpoints included mean change from baseline to 12 weeks in number of incontinence episodes/24 hours, and mean volume voided per micturition.
The efficacy of VESIcare was similar across patient age groups and gender. The mean reduction in the number of micturitions per 24 hours was significantly greater with VESIcare 5 mg (2.3; p < 0.001) and VESIcare 10 mg (2.7; p < 0.001) compared to placebo (1.4). The mean reduction in the number of incontinence episodes per 24 hours was significantly greater with VESIcare 5 mg (1.5; p < 0.001) and VESIcare 10 mg (1.8; p < 0.001) treatment groups compared to the placebo treatment group (1.1). The mean increase in the volume voided per micturition was significantly greater with VESIcare 5 mg (32.3 mL; p < 0.001) and VESIcare 10 mg (42.5 mL; p < 0.001) compared with placebo (8.5 mL).
The results for the primary and secondary endpoints in the four individual 12-week clinical studies of VESIcare are reported in Tables
7HOW SUPPLIED/STORAGE AND HANDLING
VESIcare is supplied as round, film-coated tablets, available in bottles as follows:
Each 5 mg tablet is light yellow and debossed with a logo and “150” and is available as follows:
- Bottle of 30 NDC 51248-150-01
Each 10 mg tablet is light pink and debossed with a logo and “151” and is available as follows:
- Bottle of 30 NDC 51248-151-01
- Bottle of 90 NDC 51248-151-03
Store at 25°C (77°F) with excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Angioedema and Anaphylactic Reactions
Inform patients that angioedema and anaphylactic reactions have been reported in patients treated with VESIcare. Angioedema and anaphylactic reactions may be life-threatening. Advise patients to promptly discontinue VESIcare therapy and seek immediate attention if they experience edema of the tongue or laryngopharynx, or difficulty breathing
Urinary Retention
Inform patients that VESIcare may cause urinary retention in patients with conditions associated with bladder outlet obstruction
Gastrointestinal Disorders
Inform patients that VESIcare may cause further decrease in gastrointestinal motility in patients with conditions associated with decreased gastrointestinal motility. VESIcare has been associated with constipation and dry mouth. Advise patients to contact their health care providers if they experience severe abdominal pain or become constipated for 3 or more days
Central Nervous System Effects
Because VESIcare, like other antimuscarinic agents, may cause central nervous system effects or blurred vision, advise patients to exercise caution in decisions to engage in potentially dangerous activities until the drug’s effect on the patient has been determined
Narrow-Angle Glaucoma
Inform patients that VESIcare, like other antimuscarinics, may cause worsening of the glaucoma condition in patients with narrow-angle glaucoma
Dry Skin
Inform patients that VESIcare, like other antimuscarinics, may cause dry skin due to decreased sweating. Heat prostration due to decreased sweating can occur when VESIcare is used in a hot environment
Marketed and Distributed by:
VESIcare is a registered trademark of Astellas Pharma Inc.
© 2004 – 2022 Astellas Pharma US, Inc.
361078-VES
9PRINCIPAL DISPLAY PANEL - 5 mg Carton
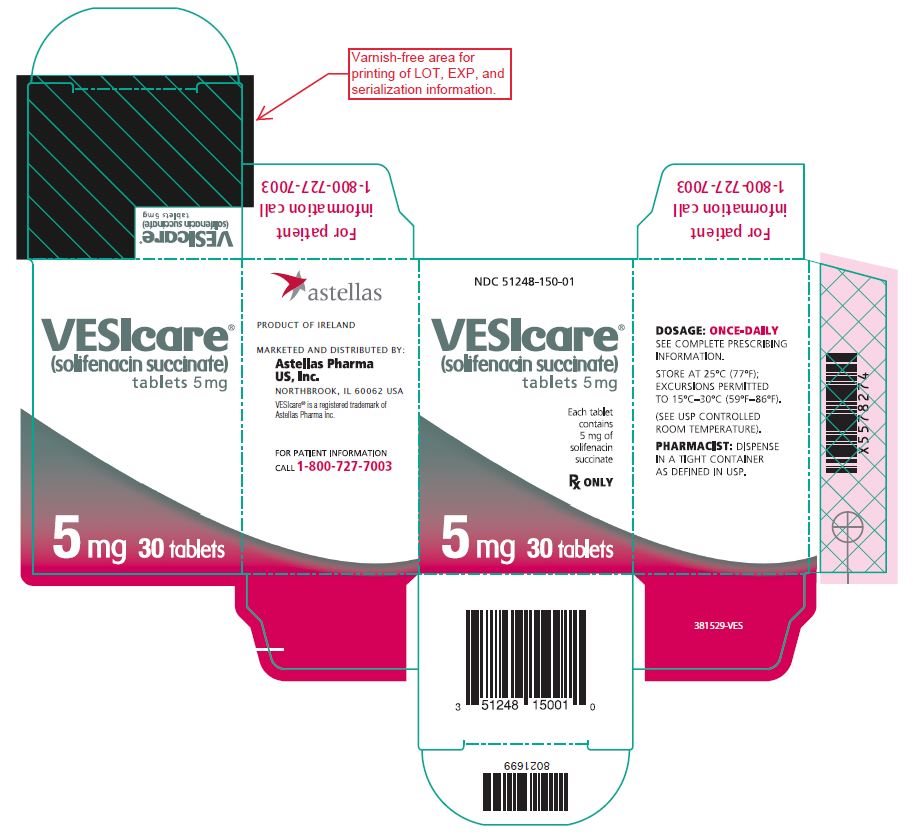
NDC 51248-150-01
VESIcare
Each tablet contains 5 mg of solifenacin succinate
Rx ONLY
5 mg 30 tablets
10PRINCIPAL DISPLAY PANEL – 10 mg Carton
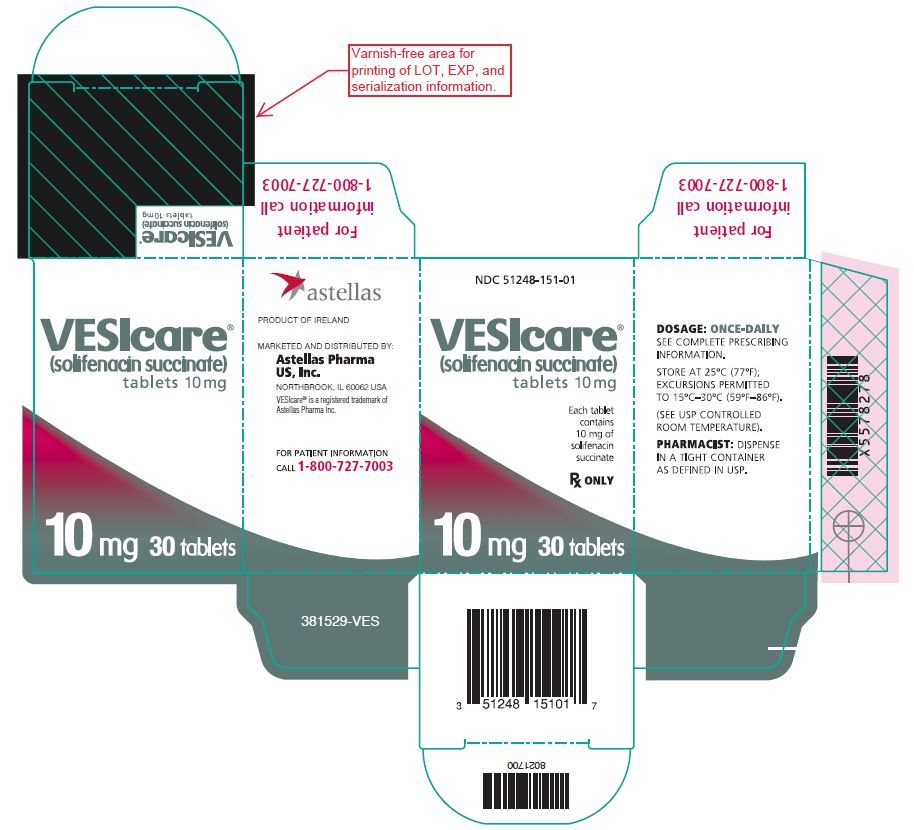
NDC 51248-151-01
VESIcare
Each tablet contains 10 mg of solifenacin succinate
Rx ONLY
10 mg 30 tablets