Brand Name
Lamzede
Generic Name
Velmanase alfa-tycv
View Brand Information FDA approval date: February 21, 2023
Form: Injection
What is Lamzede (Velmanase alfa-tycv)?
INDICA TIONS AND USAGE LAMZEDE is indicated for the treatment of non-central nervous system manifestations of alpha-mannosidosis in adult and pediatric patients. LAMZEDE is recombinant human lysosomal alpha-mannosidase indicated for the treatment of non-central nervous system manifestations of alpha-mannosidosis in adult and pediatric patients.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Real-world Analysis of Pharmacodynamic Response to Velmanase Alfa (Lamzede®) Treatment in Patients With Alpha-Mannosidosis Less Than 3 Years of Age
Summary: The goal of this observational study is to learn the effects of the drug velmanase alfa (Lamzede®) in the bodies of children under the age of 3 with Alpha-Mannosidosis. The main questions it aims to answer are: * study the effect of velmanase alfa on a marker of the disease called GlcNAc(Man)2 after one year of therapy * explore how the child's body reacts to velmanase alfa during the therapy The ...
Brand Information
Lamzede (Velmanase alfa-tycv)
WARNING: SEVERE HYPERSENSITIVITY REACTIONS
Hypersensitivity Reactions Including Anaphylaxis
Patients treated with LAMZEDE have experienced hypersensitivity reactions, including anaphylaxis. Appropriate medical support measures, including cardiopulmonary resuscitation equipment, should be readily available during LAMZEDE administration. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue LAMZEDE immediately and initiate appropriate medical treatment. In patients with severe hypersensitivity reaction, a desensitization procedure to LAMZEDE may be considered [see Warnings and Precautions (5.1)].
1INDICATIONS AND USAGE
LAMZEDE is indicated for the treatment of non-central nervous system manifestations of alpha-mannosidosis in adult and pediatric patients.
2DOSAGE FORMS AND STRENGTHS
For injection: 10 mg of velmanase alfa-tycv as a white to off-white lyophilized powder with a cake-like appearance in a single-dose vial for reconstitution.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions Including Anaphylaxis
- Infusion-Associated Reactions (IARs)
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions From Trial 1
The safety of LAMZEDE was evaluated in Trial 1, which included a total of 15 LAMZEDE-treated patients (8 adult patients aged 18-35 years old and 7 pediatric patients aged 6-17 years old; 9 male, 6 female) with alpha-mannosidosis
A serious adverse reaction of acute renal failure was reported in 1 (7%) LAMZEDE-treated patient
Table 1 lists adverse reactions that occurred in at least 2 LAMZEDE-treated patients in Trial 1.
1 “Urinary tract infection” is composed of similar terms.
Adverse Reactions from Trials 2 and 3
In Trial 2, 5 pediatric patients aged 3 to 5 years old (3 male, 2 female) with alpha-mannosidosis received LAMZEDE weekly for a mean exposure of 121 weeks
Trial 3 is an integrated analysis that pooled the cumulative databases from LAMZEDE phase 1, 2, and 3 trials in patients with alpha-mannosodosis. A total of 33 patients (20 male, 13 female) aged 6 to 35 years old (14 adults, 19 pediatric) received LAMZEDE weekly for a mean exposure of 89 weeks in adult patients and 155 weeks
One patient was withdrawn from the trial due to repeated IARs and successfully reintroduced after 89 weeks of pause.
The adverse reactions that occurred in at least 10% of patients (and are in addition to the adverse reactions already identified in Trial 1 and 2 above) included abdominal pain upper, contusion, excoriation, post-lumbar puncture syndrome, wound, weight increased, erythema, rash, and tooth extraction.
Description of Selected Adverse Reactions
AcuteRenal Failure
One patient out of 38 (3%) experienced one episode of acute renal failure. This patient paused LAMZEDE treatment for 4 weeks and acute renal failure resolved within 12 weeks of diagnosis. This patient is noted to have received the concomitant medication of ibuprofen.
Immunoglobulin A Vasculitis
One episode of immunoglobulin A vasculitis (IgAV), reported as
Seizure
One patient out of 38 (3%), with no prior history of seizures experienced more than one episode of seizures. A relationship between the occurrence of seizures in this patient and exposure to LAMZEDE cannot be excluded.
Pediatric Patients
Hypersensitivity reactions overall were reported in 36% of adult patients and 58% of pediatric patients.
Immunogenicity: Anti-Drug Antibody-Associated Adverse Reactions
Infusion-associated reactions (including anaphylaxis and severe hypersensitivity reactions) occurred in a higher incidence in LAMZEDE-treated patients who developed anti-velmanase alfa-tycv antibodies (anti-drug antibodies, ADA) compared to patients who were ADA-negative (80% versus 20%)
In Trial 1 following treatment with LAMZEDE for up to 52 weeks, 1 out of 5 ADA-positive patients developed severe hypersensitivity and this patient developed the highest ADA level among all the ADA-positive patients in the trial. In Trial 2 following treatment with LAMZEDE for up to 174 weeks, 2 out of 4 ADA-positive pediatric patients experienced IARs. In Trial 3, 3 out of 33 patients (9.1%) reported IARs; two of these patients were ADA positive (one of these two patients is described in Trial 1); one patient was ADA negative.
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of velmanase alfa outside of the United States. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac disorders: aortic valve incompetence, palpitations, tachycardia
Ear and labyrinth disorders: deafness
Eye disorders: lacrimation increased
Gastrointestinal disorders: odynophagia
General disorders and administration site conditions: asthenia, fatigue
Infections and infestations: endocarditis, staphylococcal infection, bacterial disease carrier, furuncle
Metabolism and nutrition disorders: decreased appetite
Musculoskeletal and connective tissue disorders: joint swelling, joint warmth
Nervous system disorders: ataxia, nervous system disorder, somnolence
Psychiatric disorders: psychotic disorder, agitation, encopresis, nervousness
Respiratory, thoracic and mediastinal disorders: pharyngeal edema, wheezing
Skin, subcutaneous tissue disorders: angioedema
Vascular disorders: vascular fragility, hypotension
5DESCRIPTION
Velmanase alfa-tycv, is lysosomal alpha-mannosidase produced by recombinant DNA technology in Chinese Hamster Ovary (CHO) cells. The amino acid sequence of the monomeric protein is identical to the naturally occurring human enzyme, alpha-mannosidase. Velmanase alfa-tycv has an approximate molecular weight of 130 kDa.
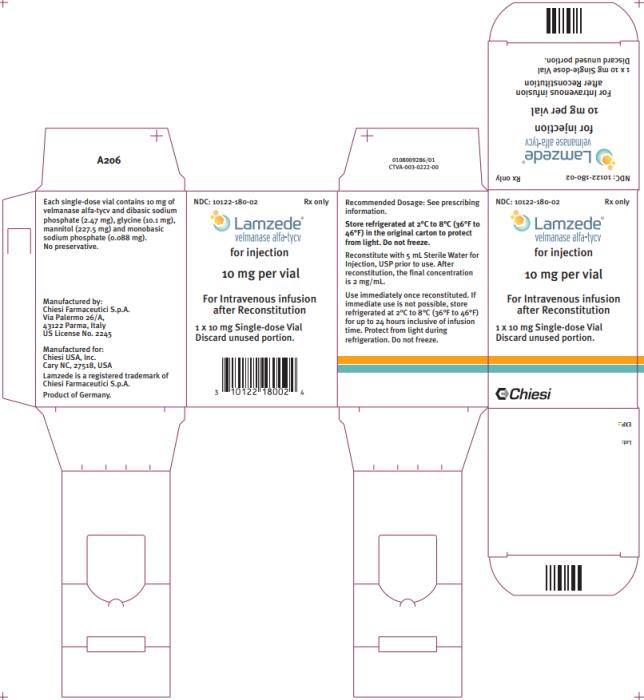
LAMZEDE (velmanase alfa-tycv) for injection is a sterile, preservative-free, white to off-white lyophilized powder with a cake-like appearance for intravenous infusion after reconstitution. Each single-dose vial contains 10 mg of velmanase alfa-tycv and the inactive ingredients dibasic sodium phosphate (2.47 mg), glycine (10.1 mg), mannitol (227.5 mg) and monobasic sodium phosphate (0.088 mg). After reconstitution with 5 mL Sterile Water for Injection, USP the resultant concentration is 2 mg/mL with pH of 7.5 ± 0.5.
6CLINICAL STUDIES
Trial 1
Trial 1 (NCT01681953) was a phase 3 multicenter, randomized, double-blinded, placebo-controlled, parallel group trial in adult and pediatric patients with alpha-mannosidosis. The trial evaluated the efficacy of LAMZEDE over 52 weeks at a dose of 1 mg/kg given weekly as an intravenous infusion. A total of 25 patients were enrolled (14 males, 11 females), including 13 adult patients (age range: ≥18 to 35 years; mean: 25 years) and 12 pediatric patients (age range: ≥6 to <18 years; mean: 11 years); all patients were White. Ethnicity data were not collected. All patients had alpha-mannosidase activity below 11% of normal and in the range of 8 to 29 µmol/h/mg at baseline. All patients but one were naïve to LAMZEDE. Fifteen patients (8 adult and 7 pediatric) received LAMZEDE and 10 patients (5 adult and 5 pediatric) received placebo. All patients completed the trial.
The efficacy results for the clinical endpoints assessed at 12 months, 3-minute stair climbing test (3MSCT), 6-minute walking test (6MWT) and forced vital capacity (FVC) (% predicted), favored the LAMZEDE group and were supported by a reduction in serum oligosaccharide concentration. The results of 3MSCT, 6MWT, FVC (% predicted), and serum oligosaccaride concentrations are presented in Table 2.
Mean = sample mean and SD = standard deviation. For each endpoint, the treatment difference in the adjusted means (95% CI) were calculated using an analysis of covariance that included baseline age, baseline value of the endpoint as covariates. Missing data of FVC (% predicted) were not imputed.
Trial2
LAMZEDE was investigated in a single arm trial in pediatric alpha-mannosidosis patients less than 6 years of age (NCT02998879). All patients had alpha-mannosidase activity below 10% of normal at baseline.The trial enrolled five patients ranging from 3.7 to 5.9 years of age, with a mean age of 4.5 years. Four patients were White, race was not recorded for 1 patient; and 3 were male and 2 were female. Patients received LAMZEDE 1 mg/kg as intravenous infusion once weekly (4 patients for 24 months, 1 patient for 40 months).
The mean (SD) absolute and percentage changes from Baseline for serum oligosaccharides at 24 months were -7.7 (4.27) μmol/L and -65.8% (23.1%) respectively.
7HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
LAMZEDE (velmanase alfa-tycv) for injection is supplied as a white to off-white lyophilized powder with a cake-like appearance in a single-dose vial. Each vial contains 10 mg of velmanase alfa-tycv. LAMZEDE is available as:
- One 10 mg single-dose vial in a carton: NDC 10122-180-02
- Five 10 mg single-dose vials in a carton: NDC 10122-180-05
- Ten 10 mg single-dose vials in a carton: NDC 10122-180-10
Storage and Handling
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze.
8PATIENT COUNSELING INFORMATION
HypersensitivityReactions Including Anaphylaxis and Infusion-Associated Reactions (IARs)
Advise the patient and caregiver that reactions related to the infusion may occur during and after LAMZEDE treatment, including anaphylactic reactions, other serious or severe hypersensitivity reactions, and IARs. Inform the patient and caregiver of the signs and symptoms of hypersensitivity reactions and IARs and to seek medical care should signs and symptoms occur
Embryo-Fetal Toxicity
LAMZEDE may cause embryo-fetal harm. Advise the pregnant female of the potential risk to the fetus. Advise a female patient and caregiver to inform their healthcare provider of a known or suspected pregnancy
Advise a female of reproductive potential to use effective contraception during treatment and for 14 days after the last dose if LAMZEDE is discontinued
Manufactured by:
Manufactured at:
Manufactured for:
LAMZEDE is a registered trademark of Chiesi Farmaceutici S.p.A.
CTVA-001-0222-00-SPL
9PRINCIPAL DISPLAY PANEL
Principle Display Panel – 1 Vial Carton
NDC 10122-180-02
Rx only
Lamzede®
Velmanase alfa-tycv
for injection
10 mg per vial
For Intravenous infusion after Reconstitution
1 x 10mg Single-dose Vial
Discard unused portion.