Brand Name
Mavenclad
Generic Name
Cladribine
View Brand Information FDA approval date: February 28, 2000
Classification: Purine Antimetabolite
Form: Injection, Tablet
What is Mavenclad (Cladribine)?
FOR USE Cladribine Injection, USP is indicated for the treatment of active Hairy Cell Leukemia as defined by clinically significant anemia, neutropenia, thrombocytopenia or disease-related symptoms.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Mavenclad (Cladribine)
WARNING: MALIGNANCIES AND RISK OF TERATOGENICITY
- Malignancies
Treatment with MAVENCLAD may increase the risk of malignancy. MAVENCLAD is contraindicated in patients with current malignancy. In patients with prior malignancy or with increased risk of malignancy, evaluate the benefits and risks of the use of MAVENCLAD on an individual patient basis. Follow standard cancer screening guidelines in patients treated with MAVENCLAD
- Risk of Teratogenicity
MAVENCLAD is contraindicated for use in pregnant women and in women and men of reproductive potential who do not plan to use effective contraception because of the potential for fetal harm. Malformations and embryolethality occurred in animals. Exclude pregnancy before the start of treatment with MAVENCLAD in females of reproductive potential. Advise females and males of reproductive potential to use effective contraception during MAVENCLAD dosing and for 6 months after the last dose in each treatment course. Stop MAVENCLAD if the patient becomes pregnant .
1INDICATIONS AND USAGE
MAVENCLAD is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include relapsing-remitting disease and active secondary progressive disease, in adults. Because of its safety profile, use of MAVENCLAD is generally recommended for patients who have had an inadequate response to, or are unable to tolerate, an alternate drug indicated for the treatment of MS
2DOSAGE FORMS AND STRENGTHS
MAVENCLAD is available as 10 mg tablets. The tablets are uncoated, white, round, biconvex, and engraved with a "C" on one side and "10" on the other side.
3CONTRAINDICATIONS
MAVENCLAD is contraindicated:
- in patients with current malignancy
- in pregnant women and in women and men of reproductive potential who do not plan to use effective contraception during MAVENCLAD dosing and for 6 months after the last dose in each treatment course. May cause fetal harm
- in patients infected with the human immunodeficiency virus (HIV
- in patients with active chronic infections (e.g., hepatitis or tuberculosis)
- in patients with a history of hypersensitivity to cladribine
- in women intending to breastfeed on a MAVENCLAD treatment day and for 10 days after the last dose
4ADVERSE REACTIONS
The following serious adverse reactions and potential risks are discussed, or discussed in greater detail, in other sections of the labeling:
- Malignancies
- Risk of Teratogenicity
- Lymphopenia
- Infections
- Hematologic Toxicity
- Graft-Versus-Host Disease With Blood Transfusion
- Liver Injury
- Hypersensitivity
- Cardiac Failure
4.1Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In the clinical trial program of cladribine in MS, 1,976 patients received cladribine for a total of 9,509 patient years. The mean time on study including follow-up was approximately 4.8 years, and approximately 24% of cladribine-treated patients had approximately 8 years of time on study including follow-up. Of these, 923 patients aged 18 to 66 years received MAVENCLAD as monotherapy at a cumulative dose of 3.5 mg per kg.
Table 2 shows adverse reactions in Study 1
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of MAVENCLAD. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Infections and Infestations: nocardiosis, varicella zoster, histoplasmosis, cryptococcosis, and toxoplasmosis
Hepatobiliary Disorders: liver injury
5OVERDOSAGE
There is no experience with overdose of MAVENCLAD. Lymphopenia is known to be dose- dependent. Particularly close monitoring of hematological parameters is recommended in patients who have been exposed to an overdose of MAVENCLAD
There is no known specific antidote to an overdose of MAVENCLAD. Treatment consists of careful observation and initiation of appropriate supportive measures. Discontinuation of MAVENCLAD may need to be considered. Because of the rapid and extensive intracellular and tissue distribution, hemodialysis is unlikely to eliminate cladribine to a significant extent.
6DESCRIPTION
MAVENCLAD contains the nucleoside metabolic inhibitor cladribine, which is a white or almost white, non-hydroscopic, crystalline powder with the molecular formula C
The structural formula isshown below:
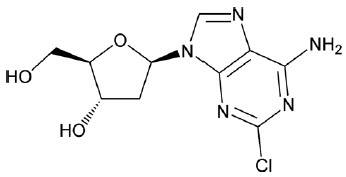
Cladribine is stable at slightly basic and at neutral pH. The main degradation pathway is hydrolysis and at acidic pH significant decomposition occurs with time. The ionization behavior of the molecule over the pH range 0 to 12 is characterized by a single pKa of approximately 1.21.
MAVENCLAD is provided as 10 mg tablets for oral use. Each MAVENCLAD 10 mg tablet contains cladribine as an active ingredient and hydroxypropyl betadex, magnesium stearate, and sorbitol as inactive ingredients.
7CLINICAL STUDIES
The efficacy of MAVENCLAD was demonstrated in a 96-week randomized, double-blind, placebo-controlled clinical study in patients with relapsing forms of MS (Study 1; NCT00213135).
Patients were required to have at least 1 relapse in the previous 12 months. The median age was 39 years (range 18 to 65) and the female-to-male ratio was approximately 2:1. The mean duration of MS prior to study enrollment was 8.7 years, and the median baseline neurological disability based on Kurtzke Expanded Disability Status Scale
1,326 patients were randomized to receive either placebo (n = 437), or a cumulative oral dosage of MAVENCLAD 3.5 mg per kg (n = 433) or 5.25 mg per kg body weight (n = 456) over the 96-week study period in 2 treatment courses. Patients randomized to the 3.5 mg per kg cumulative dose received a first treatment course at Weeks 1 and 5 of the first year and a second treatment course at Weeks 1 and 5 of the second year
The primary outcome of Study 1 was the annualized relapse rate (ARR). Additional outcome measures included the proportion of patients with confirmed disability progression, the time to first qualifying relapse, the mean number of MRI T1 Gadolinium-enhancing (Gd+) lesions, and new or enlarging MRI T2 hyperintense lesions. Disability progression was measured in terms of a 3-month sustained change in EDSS score of at least one point, if baseline EDSS score was between 0.5 and 4.5 inclusively, or at least 1.5 points if the baseline EDSS score was 0, or at least 0.5 point if the baseline EDSS score was at least 5, over a period of at least 3 months.
MAVENCLAD 3.5 mg per kg significantly lowered the annualized relapse rate. The results from Study 1 are presented in Table 4.
8REFERENCES
- "OSHA Hazardous Drugs". OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
10PRINCIPAL DISPLAY PANEL - 4 Tablet Blister Pack Carton
NDC 44087-4000-4
MAVENCLAD
Contents:
Dosage and Administration:
Separate ingestion of other oral medicines by at
Cytotoxic Agent:
Dispense with enclosed Medication Guide.
Rx Only
4 Tablets
EMD