Brand Name
Adbry
Generic Name
Tralokinumab-Ldrm
View Brand Information FDA approval date: January 02, 2022
Classification: Interleukin-13 Antagonist
Form: Injection
What is Adbry (Tralokinumab-Ldrm)?
ADBRY is indicated for the treatment of moderate-to-severe atopic dermatitis in adults and pediatric patients 12 years of age and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. ADBRY can be used with or without topical corticosteroids. ADBRY is an interleukin-13 antagonist indicated for the treatment of moderate-to-severe atopic dermatitis in adults and pediatric patients 12 years of age and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. ADBRY can be used with or without topical corticosteroids.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Adbry (tralokinumab-ldrm)
1INDICATIONS AND USAGE
ADBRY is indicated for the treatment of moderate-to-severe atopic dermatitis in adults and pediatric patients 12 years of age and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. ADBRY can be used with or without topical corticosteroids.
2DOSAGE FORMS AND STRENGTHS
ADBRY is a clear to opalescent, colorless to pale yellow solution available as:
- Injection: 150 mg/mL solution in a single-dose prefilled syringe with needle guard
- Injection: 300 mg/2 mL solution in a single-dose autoinjector
3CONTRAINDICATIONS
ADBRY is contraindicated in patients who have known hypersensitivity to tralokinumab-ldrm or any excipients in ADBRY
4ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail elsewhere in the labeling:
- Hypersensitivity
- Conjunctivitis and Keratitis
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
5OVERDOSAGE
There is no specific treatment for ADBRY overdose. In the event of overdosage, contact Poison Control (1-800-222-1222) for latest recommendations and monitor the patient for any signs or symptoms of adverse reactions and institute appropriate symptomatic treatment immediately.
6DESCRIPTION
Tralokinumab-ldrm, an interleukin-13 antagonist, is a human IgG4 monoclonal antibody. Tralokinumab-ldrm is produced in mouse myeloma cells by recombinant DNA technology, consists of 1326 amino acids, and has a molecular weight of approximately 147 kilodaltons.
ADBRY (tralokinumab-ldrm) injection is a sterile, preservative-free, clear to opalescent, colorless to pale yellow solution for subcutaneous use supplied as either a single-dose prefilled syringe with needle guard in a siliconized Type-1 clear glass syringe or a single-dose autoinjector with a siliconized Type-1 clear glass syringe inside. None of the components of the prefilled syringe, autoinjector or the needle guard are made with natural rubber latex.
Each prefilled syringe delivers 150 mg tralokinumab-ldrm in 1 mL and the inactive ingredients: acetic acid (0.3 mg), polysorbate 80 (0.1 mg), sodium acetate trihydrate (6 mg), sodium chloride (5 mg), and Water for Injection, at an approximate pH of 5.5.
Each autoinjector delivers 300 mg tralokinumab-ldrm in 2 mL and the inactive ingredients: acetic acid (0.6 mg), polysorbate 80 (0.2 mg), sodium acetate trihydrate (12 mg), sodium chloride (10 mg), and Water for Injection, at an approximate pH of 5.5.
7PATIENT COUNSELING INFORMATION
Advise the patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
8INSTRUCTIONS FOR USE
ADBRY
This Instructions for Use contains information on how to inject ADBRY.
Read this Instructions for Use before you start using the ADBRY prefilled syringe and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Keep this Instructions for Use and refer to it as needed.
Each single-dose prefilled syringe contains 150 mg of ADBRY. The ADBRY prefilled syringes are for one time use only.
Important Information You Need to Know Before Injecting ADBRY:
- Your healthcare provider should show you or your caregiver how to prepare and inject a dose of ADBRY using the prefilled syringe before you inject ADBRY for the first time. Talk to your healthcare provider if you have any questions about how to inject ADBRY the right way.
- ADBRY is given as an injection under the skin (subcutaneous injection).
- Do not inject yourself or someone else until you have been shown how to inject ADBRY the right way.
- In children 12 years of age and older, it is recommended that ADBRY be given by or under supervision of an adult.
- Talk to your healthcare provider about your prescribed dose before injecting ADBRY.
- Rotate the injection site each time you give an injection.
- The ADBRY prefilled syringe has a needle guard that will be activated to cover the needle after the injection is finished.
- Do not remove the needle cover until just before you give the injection.
- Do not share or reuse your ADBRY prefilled syringe.
- Do not inject through clothes.
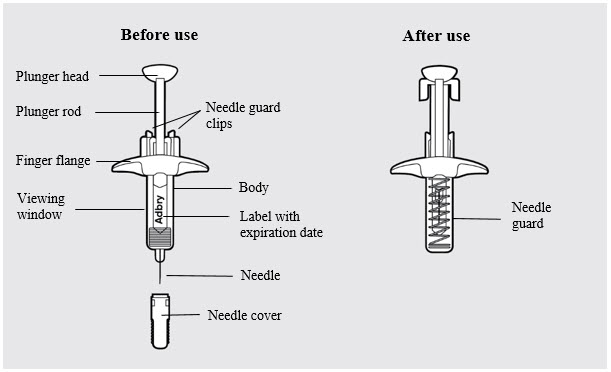
ADBRY prefilled syringe parts (see
Figure A
Step 1: Setting up ADBRY injection
Figure B
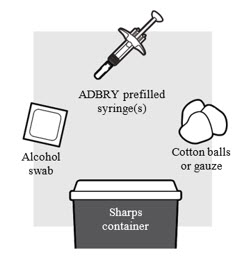
1a: Gather the supplies needed for your injection. For each ADBRY dose you will need (see
- A clean, flat, well-lit work surface, like a table
- Prescribed number of ADBRY prefilled syringe(s)
- An alcohol swab (not included in the carton)
- Clean cotton balls or gauze pads (not included in the carton)
- A puncture-resistant sharps disposal container (not included in the carton).
Figure C
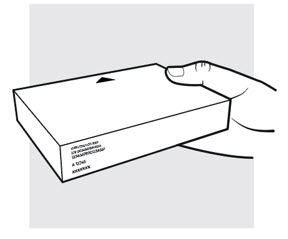
1b: Take the ADBRY prefilled syringe carton out of the refrigerator
- Check the expiration date (EXP) on the carton (see use if the expiration date on the carton has passed.
- When using the first prefilled syringe, check to make sure the seal on the ADBRY carton is intact.
- Do not use the ADBRY prefilled syringes if the seal on the carton is broken.
Figure D
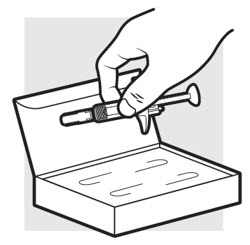
1c: Determine how many ADBRY prefilled syringes you need for your dose
If you have been prescribed a 300 mg dose, you will need 2 syringes.
If you have been prescribed a 150 mg dose, you will only need 1 syringe and the remaining syringe should be returned to the refrigerator in its carton.
- Remove the ADBRY prefilled syringe(s) by grasping the body (not the plunger rod) of the ADBRY prefilled syringe (
- Do not touch the needle guard clips to keep from activating the safety device (needle guard) too soon.
- Do not remove the needle cover on the prefilled syringe until you have reached Step 3 and are ready to inject.
Figure E

1d: Let the ADBRY prefilled syringe(s) warm up to room temperature (see
Place the ADBRY prefilled syringe(s) on the flat surface and wait 30 minutes before you inject ADBRY to let the prefilled syringe(s) warm up to room temperature 68°F to 86°F (20°C to 30°C). This will help to reduce discomfort.
- Do not microwave the prefilled syringes, run hot water over them, or leave them in direct sunlight.
- Do not shake the syringes.
- Do not remove the needle cover on the prefilled syringes until you have reached Step 3 and are ready to inject.
- Do not put the syringes back in the refrigerator after they have reached room temperature.
Figure F
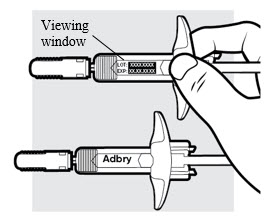
1e: Inspect the ADBRY prefilled syringe(s) (see
- Make sure ADBRY appears on the label.
- Check the expiration date printed on the syringe.
- Check the medicine through the viewing window. The medicine inside should be clear to slightly pearly and colorless to pale yellow.
- Do not use the ADBRY prefilled syringe, throw away and get a new one if:
- the expiration date printed on the syringe has passed
- the medicine is cloudy, discolored, or has particles in it
- looks damaged or has been dropped
- You may see small air bubbles in the liquid. This is normal. You do not need to do anything about it.
Step 2: Choosing and preparing injection area
Figure G
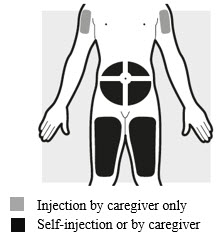
2a: Choose the area for your injection (see
- You may inject into your thighs or your stomach area (abdomen), but not within 2 inches (5 cm) of your belly button (navel).
- The upper arm can also be used if a caregiver gives the injection.
- Inject your dose into a different body area each time you inject ADBRY.
- If you need more than 1 injection for your dose, inject into the same body area, but at least 1 inch (3 cm) apart from each other.
- Do not inject where the skin is tender, damaged, bruised or scarred.
Figure H
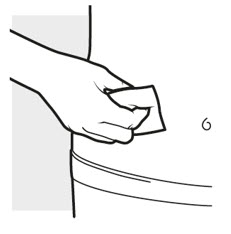
2b: Wash your hands and prepare your skin
- Wash your hands with soap and water.
- Clean the injection area with an alcohol swab using a circular motion (
Step 3: Injecting ADBRY
Figure I
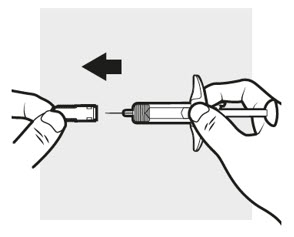
3a: Pull off the ADBRY needle cover
Hold the ADBRY prefilled syringe body with one hand, pull the needle cover straight off with your other hand (
- Do not try to recap the ADBRY prefilled syringe.
- Do not hold the plunger rod or plunger head while removing the needle cover.
- You may see a drop of liquid at the end of the needle. This is normal.
- Do not touch the needle, or let it touch any surface. If either of these occur, throw away the syringe and get a new one.
Figure J
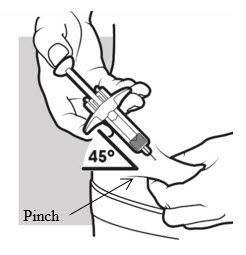
3b: Insert the needle
With one hand, gently pinch and hold a fold of skin where you cleaned the injection area. With the other hand, insert the needle completely at about a 45-degree angle into your skin (
Figure K
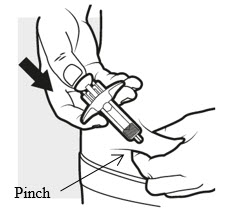
3c: Inject the medicine
Use your thumb to firmly push down the plunger head all the way down (
Figure L
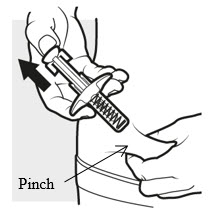
3d: Release and remove
Lift your thumb off the plunger head. The needle will automatically move back inside the syringe body and lock into place (
- Place a dry cotton ball or gauze pad over the injection site for a few seconds. Do not rub the injection site. If needed, cover the injection site with a small bandage.
- There may be a small amount of blood or liquid where you injected. This is normal.
Throw away the used ADBRY prefilled syringe in the sharps disposal container.
Step 4: Repeating for multiple injections
Figure M

If you need to give
Step 5: Disposing of ADBRY syringe(s)
Figure N
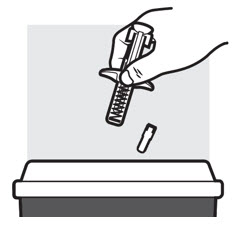
- Put the used ADBRY prefilled syringe(s) in an FDA-cleared sharps disposal container right away after use (
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not recycle your used sharps disposal container.
For more information go to www.ADBRY.com or call 1-844-692-3279. If you still have questions, call your healthcare provider.
Manufactured by: LEO Pharma A/S Industriparken 55, DK-2750 Ballerup, Denmark
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised 12/2023
9INSTRUCTIONS FOR USE
ADBRY [ad'-bree]
(tralokinumab-ldrm)
(tralokinumab-ldrm)
This Instructions for Use contains information on how to inject ADBRY.
Read this Instructions for Use before you start using the ADBRY Autoinjector and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Keep this Instructions for Use and refer to it as needed.
Each autoinjector contains 300 mg of ADBRY.
Important Information You Need to Know Before Injecting ADBRY:
- ADBRY is given as an injection under the skin (subcutaneous injection).
- Your healthcare provider should show you how to prepare and inject ADBRY using the Autoinjector before you inject ADBRY for the first time. Talk to your healthcare provider if you have any questions about how to inject ADBRY the right way.
- Do not inject yourself or someone else until you have been shown how to inject ADBRY the right way.
- The ADBRY Autoinjector is for use in adults only.
- Talk to your healthcare provider about your prescribed dose before injecting ADBRY.
- For your initial dose, you will use 2 Autoinjectors. After the initial dose, you will use 1 Autoinjector for further doses.
- Rotate the injection area with each new injection.
- The ADBRY Autoinjector has a needle guard that will be activated to cover the needle after the injection is finished.
- Do not remove the cap until just before you give the injection.
- Do not share or reuse your ADBRY Autoinjector.
- Do not inject through clothes.
ADBRY Autoinjector parts (see
Figure A

Storing ADBRY
- Store ADBRY Autoinjectors in a
- Store ADBRY Autoinjectors in the original carton and protect from light until you are ready to use them.
- ADBRY can be stored in the original carton at room temperature up to 86°F (30°C) for up to 14 days.
- Do not freeze ADBRY Autoinjectors. Do not use if they have been frozen.
- Do not shake ADBRY.
- Do not heat ADBRY.
- Do not put ADBRY into direct sunlight.
- Keep ADBRY Autoinjectors and all medicines out of the reach of children. Contains small parts.
Step 1: Setting up ADBRY injection
Figure B

1a: Gather the supplies needed for your injection.
- A clean, flat, well-lit work surface, like a table
- 1 ADBRY Autoinjector
- An alcohol swab (not included in the carton)
- Clean cotton balls or gauze pads (not included in the carton)
- A puncture-resistant sharps disposal container (not included in the carton)
1b: Take the ADBRY Autoinjector carton out of the refrigerator
Figure C
Check the expiration date (EXP) on the carton (
Check to make sure the seal on the carton is intact.
1c: Remove the ADBRY Autoinjector from the carton
Figure D
Remove 1 autoinjector from the carton (
- When using the first autoinjector, put the carton with the remaining autoinjector back in the refrigerator.
- Do not remove the cap on the autoinjector until you have reached Step 3 and are ready to inject.
1d: Let the ADBRY Autoinjector warm up to room temperature
Figure E

Place the autoinjector on the flat surface and wait at least 45 minutes before you inject ADBRY to let the autoinjector warm up to room temperature 68°F to 86°F (20°C to 30°C) (
- Do not microwave the autoinjector, run hot water over it, or leave it in direct sunlight.
- Do not shake the autoinjector.
- Do not put the autoinjector back in the refrigerator after it has reached room temperature.
1e: Inspect the ADBRY Autoinjector (see
Figure F
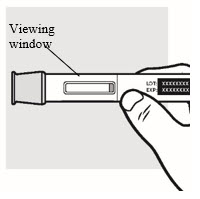
- Make sure ADBRY appears on the label.
- Check the expiration date (EXP) printed on the autoinjector label.
- Check the medicine through the viewing window. The medicine inside should be clear to slightly pearly and colorless to pale yellow.
- You may see small air bubbles in the liquid. This is normal. You do not need to do anything about it.
Do not use the ADBRY Autoinjector, throw away in a sharps disposal container and get a new one if:
- the expiration date printed on the autoinjector label has passed.
- the medicine is cloudy, discolored, or has particles in it.
- it looks damaged or has been dropped.
Step 2: Choosing and preparing injection area
Figure G
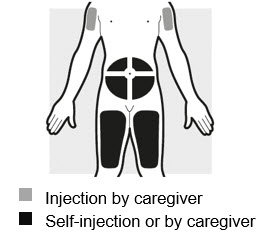
- You may inject into:
- Do not inject within 2 inches (5 cm) of your belly button (navel).
- Rotate the body area with each injection. Do not use the same body area 2 times in a row.
- Do not inject where the skin is tender, damaged, bruised or scarred.
2b: Wash your hands and prepare your skin
Figure H
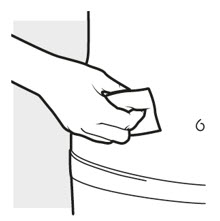
- Wash your hands with soap and water.
- Clean the injection area with an alcohol swab using a circular motion (
Step 3: Injecting ADBRY
Figure I
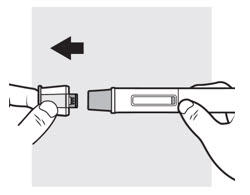
Hold the autoinjector with one hand, pull the cap straight off with your other hand (
- Do not try to recap the autoinjector. This could cause the injection to happen too soon or damage the needle.
- Do not try to touch or push on the needle guard with your finger. This could cause needle stick injury.
3b: Place the ADBRY Autoinjector at the injection area as shown in Figure J so that you can see the viewing window
Figure J
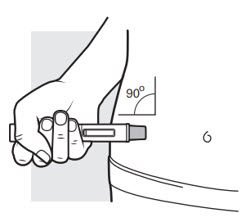
You can gently pinch the skin where you cleaned the injection area or give the injection without pinching the skin. Follow your healthcare provider´s instructions on how to inject.
- Place the needle guard of the autoinjector flat against your skin (90-degree angle) at the injection area you have cleaned. Make sure you can see the viewing window (
- Do not change the position of the autoinjector after the injection has started.
If the autoinjector is removed too soon, you may see a stream of medicine coming from the autoinjector. If this happens you may not have received your full dose. Call your healthcare provider.
3c: Press down on the ADBRY Autoinjector and hold pressure
Figure K
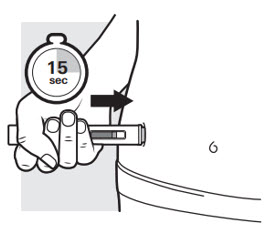
Press the autoinjector down firmly and hold it in place (
The yellow plunger will move to the bottom of the viewing window as the medicine is being injected.
You will hear a second
Keep pressing.
3d: Continue to press down for another 5 seconds
Figure L
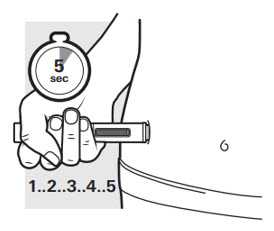
After the second "click", continue to press the autoinjector firmly against your skin for 5 seconds to make sure you get your full dose (
3e: Remove the ADBRY Autoinjector
Figure M
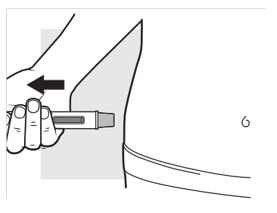
Pull the autoinjector straight away from the injection area (
Place a dry cotton ball or gauze pad over the injection area for a few seconds.
There may be a small amount of blood or liquid where you injected. This is normal. If needed, cover the injection area with a small bandage.
3f: Check the viewing window
Figure N
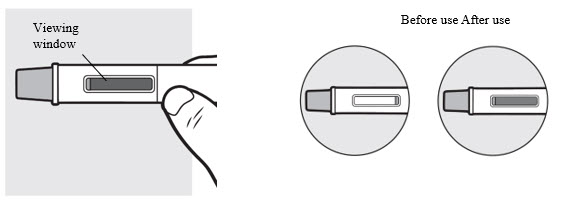
Check the viewing window to make sure all the liquid has been injected (
If the yellow plunger does not fill the viewing window you may not have received the full dose. If this happens or if you have any other concerns, call your healthcare provider and call LEO Pharma at 877-494-4536 to report a product quality complaint.
Step 4: Throwing away (Disposing of) ADBRY Autoinjectors
Figure O
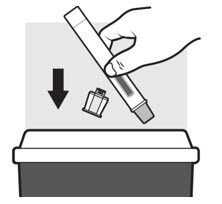
Put the used ADBRY Autoinjectors in an FDA-cleared sharps disposal container right away after use (
- Do not throw away the ADBRY Autoinjectors in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used ADBRY Autoinjectors. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
For more information go to www.ADBRY.com or call 1-844-692-3279. If you still have questions, call your healthcare provider.
Manufactured by: LEO Pharma A/S Industriparken 55, DK-2750 Ballerup, Denmark
ADBRY
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: June 2024
10PRINCIPAL DISPLAY PANEL - 150 mg/mL Syringe Carton - NDC 50222-346-02
Adbry
NDC 50222-346-02
x 2
For Subcutaneous Use Only
One 1-mL single-dose prefilled syringe equals 150 mg
Carton contains:
- Two 1-mL single-dose prefilled syringes with needle guard
- Prescribing Information
- Instructions for Use and Patient Information
Store refrigerated at 36°F to 46°F (2°C to 8°C) in
LEO

11PRINCIPAL DISPLAY PANEL - 150 mg/mL Syringe Carton - NDC 50222-346-04
Adbry
NDC 50222-346-04
x 4
For Subcutaneous Use Only
One 1-mL single-dose prefilled syringe equals 150 mg
Multipack contains:
- Two cartons
- Each carton contains two 1-mL single-dose prefilled syringes
Store refrigerated at 36°F to 46°F (2°C to 8°C) in
LEO

12PRINCIPAL DISPLAY PANEL - 300 mg/2 mL Autoinjector Carton - NDC 50222-350-01
Adbry
NDC 50222-350-01
For Subcutaneous Use Only
One 2 mL Single-dose Autoinjector equals one dose of 300 mg
Carton contains:
- One Single-dose Autoinjector
- Prescribing Information
- Instructions for Use and Patient Information
Store refrigerated at 36°F to 46°F (2°C to 8°C) in
LEO

13PRINCIPAL DISPLAY PANEL - 300 mg/2 mL Autoinjector Carton - NDC 50222-350-02
Adbry
NDC 50222-350-02
x 2
For Subcutaneous Use Only
One 2 mL Single-dose Autoinjector equals one dose of 300 mg
Carton contains:
- Two Single-dose Autoinjectors
- Prescribing Information
- Instructions for Use and Patient Information
Store refrigerated at 36°F to 46°F (2°C to 8°C) in
LEO

