Truqap
What is Truqap (Capivasertib)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This study will evaluate the safety, tolerability, and preliminary effectiveness of AMXT 1501 and DFMO when combined with standard treatments for advanced solid tumors. The trial includes two groups: * Cohort 1: Patients with ER+ / HER2- breast cancer receiving fulvestrant and capivasertib * Cohort 2: Patients with unresectable or metastatic cutaneous melanoma receiving pembrolizumab The Phase 1b ...
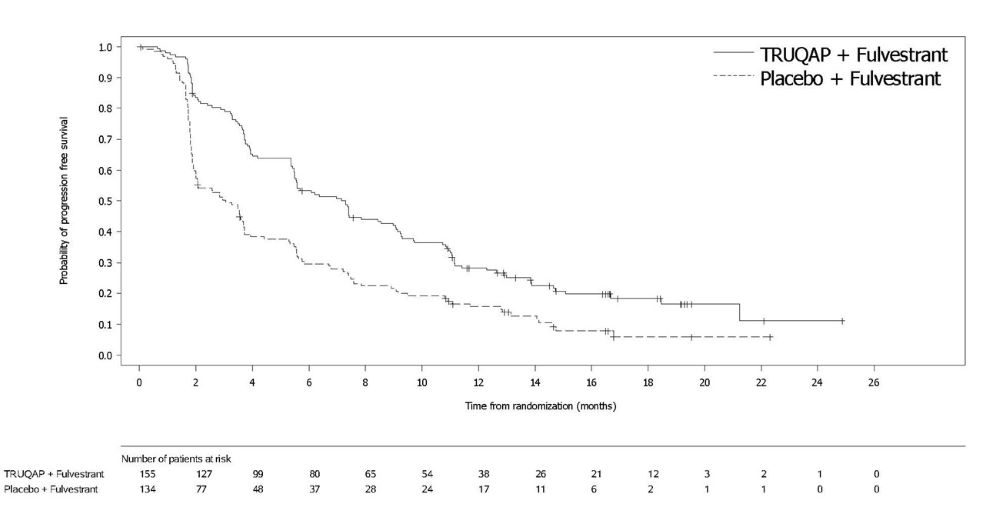
Summary: The purpose of this study is to evaluate the effectiveness and safety of capivasertib + fulvestrant treatment administration in patients with locally advanced (inoperable) or metastatic HR+ / HER2- breast cancer with PIK3CA/AKT1/PTEN-altered following recurrence or progression on or after endocrine therapy and CDK4/6 inhibitor.
Summary: This is a multicentre phase-III-trial to evaluate the use of capivasertib in patients with HR+/HER2- advanced breast cancer and progression on prior endocrine-based treatment. The goal of this study is 1. To evaluate benefit of capivasertib regarding time to next treatment (TTNT1) - i.e., time on treatment with capivasertib. 2. To evaluate the benefits of patient reported outcome(PRO)-adherence re...
Related Latest Advances
Brand Information
- 160 mg: beige film-coated, round, biconvex tablets debossed with ‘CAV’ above ‘160’ on one side and plain on the reverse.
- 200 mg: beige film-coated, capsule-shaped, biconvex tablets debossed with ‘CAV 200’ on one side and plain on the reverse.
- Hyperglycemia
- Diarrhea
- Cutaneous Adverse Reactions
- The original bottle.
- A USP equivalent tight container. Instruct patients to keep the unused tablets in the container at 20°C to 25°C (68°F to 77°F) and discard after 45 days.
- Advise females to inform their healthcare provider of a known or suspected pregnancy
- Advise females of reproductive potential to use effective contraception during treatment with TRUQAP and for 1 month after the last dose
- Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TRUQAP and for 4 months after the last dose
- Refer to the Full Prescribing Information of fulvestrant for pregnancy and contraception information.