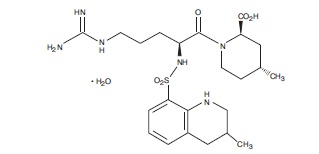
Adverse Reactions in Patients with HIT (With or Without Thrombosis)
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following safety information is based on all 568 patients treated with argatroban in Study 1 and Study 2. The safety profile of the patients from these studies is compared with that of 193 historical controls in which the adverse reactions were collected retrospectively. Adverse reactions are separated into hemorrhagic and non-hemorrhagic reactions.
Major bleeding was defined as bleeding that was overt and associated with a hemoglobin decrease ≥ 2 g/dL, that led to a transfusion of ≥ 2 units, or that was intracranial, retroperitoneal, or into a major prosthetic joint. Minor bleeding was overt bleeding that did not meet the criteria for major bleeding.
Table 4 gives an overview of the most frequently observed hemorrhagic reactions, presented separately by major and minor bleeding, sorted by decreasing occurrence among argatroban-treated patients with HIT (with or without thrombosis).
* with or without thrombosis
Table 5 gives an overview of the most frequently observed non-hemorrhagic reactions sorted by decreasing frequency of occurrence (≥2%) among argatroban-treated HIT/HITTS patients.
a) Patients may have experienced more than 1 adverse reaction.
Adverse Reactions in Patients with or at Risk for HIT Undergoing PCI
The following safety information is based on 91 patients initially treated with argatroban and 21 patients subsequently re-exposed to argatroban for a total of 112 PCIs with argatroban anticoagulation. Adverse reactions are separated into hemorrhagic (Table 6) and non-hemorrhagic (Table 7) reactions.
Major bleeding was defined as bleeding that was overt and associated with a hemoglobin decrease ≥5 g/dL, that led to a transfusion of ≥2 units, or that was intracranial, retroperitoneal, or into a major prosthetic joint. The rate of major bleeding events in patients treated with argatroban in the PCI trials was 1.8%.
a) Patients may have experienced more than 1 adverse reaction.
Table 7 gives an overview of the most frequently observed non-hemorrhagic adverse reactions (>2%), sorted by decreasing frequency of occurrence among argatroban-treated PCI patients.
a) Patients may have experienced more than 1 adverse reaction.
There were 22 serious adverse reactions in 17 PCI patients (19.6% in 112 interventions). Table 8 lists the serious adverse reactions occurring in argatroban-treated patients with or at risk for HIT undergoing PCI.
a) Individual reactions may also have been reported elsewhere (see Table 6 and 7).
Intracranial Bleeding in Other Populations
Increased risks for intracranial bleeding have been observed in investigational studies of argatroban for other uses. In a study of patients with acute myocardial infarction receiving both argatroban and thrombolytic therapy (streptokinase or tissue plasminogen activator), the overall frequency of intracranial bleeding was 1% (8 out of 810 patients). Intracranial bleeding was not observed in 317 subjects or patients who did not receive concomitant thrombolysis
The safety and effectiveness of argatroban for cardiac indications other than PCI in patients with HIT have not been established. Intracranial bleeding was also observed in a prospective, placebo-controlled study of argatroban in patients who had onset of acute stroke within 12 hours of study entry. Symptomatic intracranial hemorrhage was reported in 5 of 117 patients (4.3%) who received argatroban at 1 to 3 mcg/kg/min and in none of the 54 patients who received placebo. Asymptomatic intracranial hemorrhage occurred in 5 (4.3%) and 2 (3.7%) of the patients, respectively.
Allergic Reactions
One hundred fifty-six allergic reactions or suspected allergic reactions were observed in 1,127 individuals who were treated with argatroban in clinical pharmacology studies or for various clinical indications. About 95% (148/156) of these reactions occurred in patients who concomitantly received thrombolytic therapy (e.g., streptokinase) or contrast media.
Allergic reactions or suspected allergic reactions in populations other than patients with HIT (with or without thrombosis) include (in descending order of frequency):
- Airway reactions (coughing, dyspnea): 10% or more
- Skin reactions (rash, bullous eruption): 1 to <10%
- General reactions (vasodilation): 1 to 10%
Limited data are available on the potential formation of drug-related antibodies. Plasma from 12 healthy volunteers treated with argatroban over 6 days showed no evidence of neutralizing antibodies. No loss of anticoagulant activity was noted with repeated administration of argatroban to more than 40 patients.