Carbaglu
What is Carbaglu (Carglumic)?
Related Clinical Trials
Summary: To obtain short-term and long-term clinical safety information, in pediatric and adult patients with PA and MMA treated with Carbaglu®.
Summary: The purpose of this study is to conduct post-marketing surveillance of carglumic acid (Carbaglu) to obtain long-term clinical safety information. Carglumic acid was approved by the United States Food and Drug Administration (FDA) for treatment of acute hyperammonemia due to N-acetylglutamate synthase (NAGS) deficiency. Much of the FDA-required data is already collected through the Longitudinal Stu...
Summary: This is a prospective mixed-design study focused on the long-term management of propionic aciduria (PA) and methylmalonic aciduria (MMA) with N-carbamylglutamate (NCG) maintenance therapy. Treatment characteristics, clinical outcomes, and healthcare utilization data of patients diagnosed PA or MMA treated \>6 months therapy with NCG are collected at baseline, 12 months, 18 months, 36 months and 54...
Related Latest Advances
Brand Information
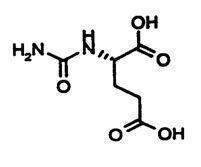
CARBAGLU is a white and elongated 200 mg tablet for oral suspension, functionally scored with 3 lines for splitting into 4 equal portions, and coded "C" on one side.
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original unopened bottle.
- Store at room temperature between 15°C and 30°C (59°F and 86°F). Do not refrigerate.
- Keep the bottle tightly closed between openings in order to protect from moisture.
- Write the date of opening on the bottle.
- Do not use CARBAGLU after the expiration date stated on the bottle.
- Discard bottle one month after first opening.
- Disperse CARBAGLU tablets in water. Do not swallow whole or crushed.
- Take CARBABLU immediately before meals or feedings.
- CARBAGLU tablets dispersed in water can be administered orally or via a nasogastric tube or gastrostomy tube as described in the
- Store UNOPENED bottle in a refrigerator at 2°C to 8°C (36°F to 46°F).
- After first opening of the bottle: do not refrigerate, store at room temperature between 15°C and 30°C (59°F and 86°F). Keep the bottle tightly closed in order to protect from moisture. Write the date of opening on the bottle.
- Discard bottle one month after first opening. Do not use CARBAGLU after the expiration date stated on the bottle.
Advise women with NAGS deficiency who are exposed to CARBAGLU during pregnancy that there is a pregnancy surveillance program that monitors pregnancy outcomes.

Carbaglu® 200 mg
carglumic acid
tablets for oral suspension
60 Tablets
Rx only
Before opening, store refrigerated at
2°C – 8°C (36°F – 46°F). Do not refrigerate.
Do not store above 30°C (86°F).
Discard one month after first opening
Date of first opening.
4022649 5/1344

Carbaglu® 200 mg
carglumic acid
tablets for oral suspension
60 Tablets
Rxonly
Disperse CARBAGLU tablets in water.
Do not swallow whole or crushed.
Supplied by:
Recordati Rare Diseases
Puteaux, France
Distributed by:
Recordati Rare Diseases Inc.
Lebanon, NJ 08833 USA
RECORDATI
Group

Carbaglu® 200 mg
carglumic acid
tablets for oral suspension
5 Tablets
Rxonly
Before opening, store refrigerated at
2°C – 8°C (36°F – 46°F). Do not refrigerate.
Do not store above 30°C (86°F).
Discard one month after first opening
Date of first opening.
4022647 5/1142

Carbaglu® 200 mg
carglumic acid
tablets for oral suspension
5 Tablets
Rxonly
Disperse CARBAGLU tablets in water.
Do not swallow whole or crushed.
Supplied by:
Recordati Rare Diseases
Puteaux, France
Distributed by:
Recordati Rare Diseases Inc.
Lebanon, NJ 08833 USA
RECORDATI
Group
