Brand Name
Haldol Decanoate
Generic Name
Decanoate
View Brand Information FDA approval date: January 14, 1986
Classification: Typical Antipsychotic
Form: Injection
What is Haldol Decanoate (Decanoate)?
Haloperidol decanoate injection is indicated for the treatment of patients with schizophrenia who require prolonged parenteral antipsychotic therapy.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Haldol Decanoate (Haloperidol Decanoate)
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. HALDOL DECANOATE is not approved for the treatment of patients with dementia-related psychosis
1INDICATIONS AND USAGE
HALDOL DECANOATE is indicated for the treatment of schizophrenia in adults who were previously taking a stable dosage of an immediate-release oral haloperidol product.
2DOSAGE FORMS AND STRENGTHS
Injection:
- Haloperidol 50 mg/mL (present as haloperidol decanoate) is a clear, yellow to light amber viscous liquid, free from visible foreign material, in a single-dose ampule
- Haloperidol 100 mg/mL (present as haloperidol decanoate) is a clear, yellow to light amber viscous liquid, free from visible foreign material, in a single-dose ampule
3CONTRAINDICATIONS
HALDOL DECANOATE is contraindicated in patients with:
- Severe toxic central nervous system depression or comatose states from any cause.
- Known hypersensitivity to haloperidol or any components of HALDOL DECANOATE. Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been reported in patients treated with haloperidol
- Parkinson's disease
- Dementia with Lewy bodies
4ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Sudden Death, Torsades de Pointes, and QTc Interval Prolongation
- Tachycardia and Hypotension
- Tardive Dyskinesia
- Neuroleptic Malignant Syndrome
- Seizures
- Hypersensitivity Reactions
- Leukopenia, Neutropenia, and Agranulocytosis
- Hyperprolactinemia
- Risk of Severe Neurotoxicity in Patients with Thyrotoxicosis
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions Identified in Clinical Trials with HALDOL DECANOATE
The data described below reflect exposure to 15 mg to 500 mg (1.7 times the maximum recommended dosage) of HALDOL DECANOATE monthly in 13 clinical trials of 410 adult patients with schizophrenia or an unapproved condition. These clinical trials comprised of:
- 1 double-blind, active comparator-controlled trial with fluphenazine decanoate (Trial 1).
- 2 trials comparing HALDOL DECANOATE to oral haloperidol (Trials 2 and 3).
- 9 open-label trials.
- 1 dose-response trial.
The most common adverse reactions that occurred in ≥5% of HALDOL DECANOATE-treated patients in Trial 1 were Parkinsonism and oculogyric crisis.
Adverse reactions that occurred in ≥1% of HALDOL DECANOATE-treated patients in Trial 1 are shown in Table 2. Trial 1 was not designed to evaluate meaningful comparisons of the incidence of adverse reactions in the HALDOL DECANOATE and fluphenazine decanoate treatment groups.
Less common adverse reactions (<1%) that occurred in Trial 1 and other adverse reactions that occurred in Trials 2 and 3, and open-label and dose-response clinical trials of HALDOL DECANOATE are listed below.
- Cardiac Disorders:Tachycardia
- Endocrine Disorders:Hyperprolactinemia
- Eye Disorders:Vision blurred
- Gastrointestinal Disorders:Constipation, Dry mouth, Salivary hypersecretion
- General Disorders and Administration Site Conditions:Weight increased, Injection site reaction
- Musculoskeletal and Connective Tissue Disorders:Muscle rigidity
- Nervous System Disorders:Dyskinesia, Dystonia, Cogwheel rigidity, Hypertonia, Masked facies, Sedation, Somnolence
- Reproductive System Disorders:Erectile dysfunction
Adverse Reactions Identified in Clinical Trials with Immediate-Release Haloperidol Products
Based on clinical trials with immediate-release haloperidol products that included 1,579 patients, the following adverse reactions were reported:
- Musculoskeletal and Connective Tissue Disorders:Torticollis, Trismus, Muscle twitching
- Nervous System Disorders:Neuroleptic malignant syndrome, Tardive dyskinesia, Bradykinesia, Hyperkinesia, Hypokinesia, Dizziness, Nystagmus
- Psychiatric Disorders:Loss of libido, Restlessness
- Reproductive System and Breast Disorders:Amenorrhea, Galactorrhea, Dysmenorrhea, Menorrhagia, Breast discomfort
- Skin and Subcutaneous Tissue Disorders:Acneiform skin reactions
- Vascular Disorders:Hypotension, Orthostatic hypotension
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of haloperidol, including HALDOL DECANOATE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic System Disorders:Pancytopenia, Agranulocytosis, Thrombocytopenia, Leukopenia, Neutropenia
- Cardiac Disorders:Ventricular fibrillation, Torsade de pointes, Ventricular tachycardia, Extrasystoles, QTc interval prolongation
- Endocrine Disorders:Inappropriate antidiuretic hormone secretion
- Gastrointestinal Disorders:Vomiting, Nausea
- General Disorders and Administration Site Conditions:Sudden death, Face edema, Edema, Hyperthermia, Hypothermia, Injection site abscess, Weight decreased
- Hepatobiliary Disorders:Acute hepatic failure, Hepatitis, Cholestasis, Jaundice, Liver function test abnormal
- Immune System Disorders:Anaphylactic reaction, Hypersensitivity
- Metabolic and Nutritional Disorders:Hypoglycemia
- Musculoskeletal and Connective Tissue Disorders:Rhabdomyolysis
- Nervous System Disorders:Convulsion, Opisthotonus, Tardive dystonia
- Pregnancy, Puerperium and Perinatal Conditions:Neonatal drug withdrawal syndrome
- Psychiatric Disorders:Agitation, Confusional state, Depression, Insomnia
- Renal and Urinary Disorders:Urinary retention
- Reproductive System and Breast Disorders:Priapism, Gynecomastia
- Respiratory, Thoracic and Mediastinal Disorders:Laryngeal edema, Bronchospasm, Laryngospasm, Dyspnea
- Skin and Subcutaneous Tissue Disorders:Angioedema, Dermatitis exfoliative, Hypersensitivity vasculitis, Photosensitivity reaction, Urticaria, Pruritus, Rash, Hyperhidrosis
5OVERDOSAGE
Reported overdose signs and symptoms are those resulting from an exaggeration of the drug's known pharmacologic effects (e.g., drowsiness and sedation, tachycardia and hypotension) and adverse reactions, the most prominent of which would be: 1) severe extrapyramidal symptoms, 2) hypotension, or 3) sedation. Patients may appear comatose with respiratory depression and hypotension which could be severe enough to produce a shock-like state. Extrapyramidal reactions may be manifested by muscular weakness or rigidity and a generalized or localized tremor, as demonstrated by the akinetic or agitans types, respectively. The risk of QTc interval prolongation and torsade de pointes should be considered
Management of Overdose
There is no specific antidote for a haloperidol overdose.
- Should hypotension occur and a vasopressor be required, epinephrine must not be used since HALDOL DECANOATE may block its vasopressor activity, and paradoxical further lowering of the blood pressure may occur. Instead, metaraminol, phenylephrine or norepinephrine should be used.
- In case of severe extrapyramidal reactions, antiparkinson drugs should be administered, and should be continued for several weeks, and then withdrawn gradually as extrapyramidal symptoms may emerge if discontinued abruptly.
- Monitor ECG and vital signs for signs of QTc interval prolongation or dysrhythmias and continue monitoring until the dysrhythmias resolve and the haloperidol-induced QTc interval prolongation resolves.
- Dialysis is not recommended in the treatment of overdose because it removes only very small amounts of haloperidol.
Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
6DESCRIPTION
HALDOL DECANOATE (haloperidol decanoate injection) is the decanoate ester of haloperidol, for intramuscular use. Haloperidol is a typical antipsychotic. The structural formula of haloperidol decanoate is 4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl] piperidin-4-yl decanoate:
The molecular formula is C
Each mL of HALDOL DECANOATE contains:
- 50 mg of haloperidol (present as 70.5 mg of haloperidol decanoate) in a sesame oil vehicle (0.85 g/mL), with 15 mg/mL benzyl alcohol as a preservative.
- 100 mg of haloperidol (present as 141 mg of haloperidol decanoate) in a sesame oil vehicle (0.85 g/mL), with 15 mg/mL benzyl alcohol as a preservative.
7HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
HALDOL DECANOATE (haloperidol decanoate injection) is a clear, yellow to light amber viscous liquid, free from visible foreign material and available as:
- haloperidol 50 mg/mL (present as haloperidol decanoate)
- haloperidol 100 mg/mL (present as haloperidol decanoate)
8PATIENT COUNSELING INFORMATION
Sudden Death, Torsades de Pointes, and QTc Interval Prolongation
Inform patients that there have been reports of sudden death, torsades de Pointes, and QTc interval prolongation in haloperidol-treated patients. Advise patients or caregivers to seek immediate medical attention if they suspect or develop signs or symptoms associated with the clinical consequences of QTc interval prolongation
Tardive Dyskinesia
Inform patients that tardive dyskinesia (TD) may develop with HALDOL DECANOATE. Counsel patients on the signs and symptoms of tardive dyskinesia and to contact their healthcare provider if these abnormal movements occur
Neuroleptic Malignant Syndrome
Counsel patients about a potentially fatal adverse reaction, Neuroleptic Malignant Syndrome (NMS), that has been reported with administration of antipsychotic drugs. Advise patients, family members, or caregivers to contact the health care provider or to report to the emergency room if they experience signs and symptoms of NMS
Hypersensitivity Reactions
Inform patients of the potential risk of hypersensitivity reactions. Advise patients to stop taking HALDOL DECANOATE and seek immediate attention if signs or symptoms of a hypersensitivity reaction occur
Falls
Inform patients that HALDOL DECANOATE can cause somnolence, orthostatic hypotension, motor instability and sensory abnormality that may lead to falls. Advise patients to notify their healthcare provider if any of these symptoms occur
Potential for Cognitive and Motor Impairment
Inform patients of the risk and advise them to not drive a motor vehicle or operate hazardous machinery until they are reasonably certain that treatment with HALDOL DECANOATE does not impair their cognitive and motor functions
Leukopenia/Neutropenia
Advise patients with a pre-existing low WBC or a history of drug induced leukopenia or neutropenia that they should have their CBC monitored while taking HALDOL DECANOATE
Hyperprolactinemia
Counsel patients on signs and symptoms of hyperprolactinemia that may be associated with chronic use of HALDOL DECANOATE. Advise the patients to seek medical attention if they experience any of the following: amenorrhea, galactorrhea, erectile dysfunction or gynecomastia
Pregnancy
Advise pregnant patients to notify their health care provider if they become pregnant or intend to become pregnant during treatment with HALDOL DECANOATE. Advise patients that HALDOL DECANOATE exposure during the third trimester of pregnancy may cause adverse effects in the neonate, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and decreased feeding
Lactation
Advise breastfeeding patients using HALDOL DECANOATE to monitor infants for excess sedation, irritability, poor feeding, and extrapyramidal symptoms (tremors and abnormal muscle movements) and to seek medical care if they notice these signs
Infertility
Advise females and males of reproductive potential that HALDOL DECANOATE may impair fertility due to an increase in serum prolactin levels
Drug Interactions
Advise patients to inform their health care provider before starting or discontinuing a prescription drug, nonprescription drug, or supplement
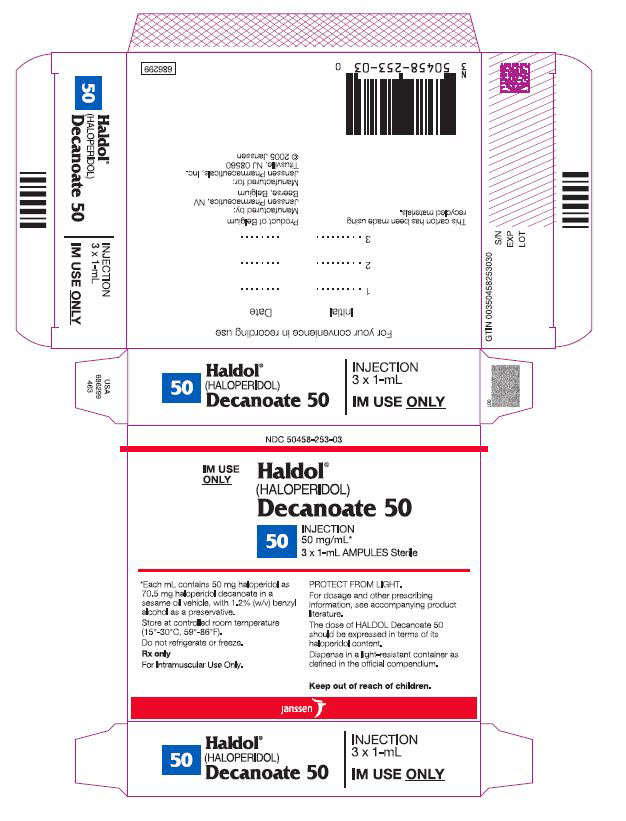
9PRINCIPAL DISPLAY PANEL - 50 mg/mL box
NDC 50458-253-03
IM Use
Haldol
(HALOPERIDOL)
Decanoate 50
(HALOPERIDOL)
Decanoate 50
50 INJECTION
50 mg/mL*
50 mg/mL*
3 x 1-mL AMPULS Sterile
*Each mL contains 50 mg haloperidol
Store at controlled room temperature
Rx only.
For Intramuscular Use Only.
PROTECT FROM LIGHT.
For dosage and other prescribing
The dose of HALDOL Decanoate 50
Dispense in a light-resistant
Keep out of reach of children.
Janssen
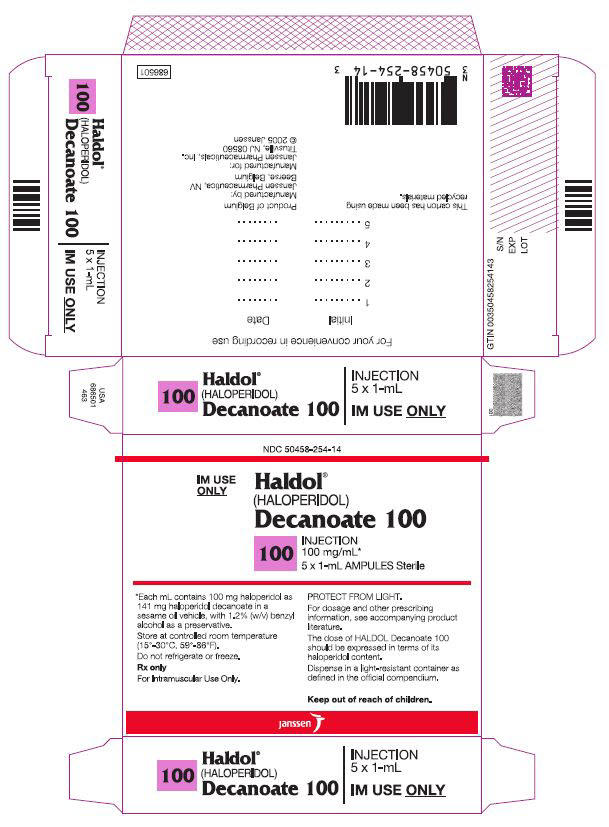
10PRINCIPAL DISPLAY PANEL - 100 mg/mL Box
NDC 50458-254-14
IM Use
Haldol
(HALOPERIDOL)
Decanoate 100
(HALOPERIDOL)
Decanoate 100
100 INJECTION
100 mg/mL*
100 mg/mL*
5 x 1-mL AMPULS Sterile
*Each mL contains 100 mg haloperidol
Store at controlled room temperature
Rx only.
For Intramuscular Use Only.
PROTECT FROM LIGHT.
For dosage and other prescribing
The dose of HALDOL Decanoate 100
Dispense in a light-resistant
Keep out of reach of children.
Janssen