Zilbrysq
What is Zilbrysq (Zilucoplan)?
Living with generalized myasthenia gravis (gMG) can turn ordinary activities like speaking, swallowing, or even holding up one’s head into exhausting challenges. The unpredictable muscle weakness and fatigue caused by this autoimmune disease can deeply affect daily life, independence, and emotional well-being. Zilbrysq (zilucoplan) offers a new way forward for adults living with this difficult condition.
Zilbrysq is a prescription medication used to treat generalized myasthenia gravis in adults who test positive for acetylcholine receptor (AChR) antibodies, the most common type of gMG. It belongs to a class of drugs known as complement inhibitors, which target part of the immune system responsible for attacking the body’s own nerve-muscle communication.
Approved by the U.S. Food and Drug Administration (FDA) in 2023, Zilbrysq represents a newer, targeted therapy designed for patients who need better control of their symptoms despite standard treatments. Its introduction marks an important advancement in personalized care for gMG offering both symptom relief and convenience through self-administration at home.
What does Zilbrysq do?
Zilbrysq is used to treat generalized myasthenia gravis, a chronic autoimmune disorder where the immune system disrupts communication between nerves and muscles. This disruption leads to muscle weakness that can affect the eyes, face, throat, limbs, and respiratory muscles.
By controlling an overactive immune response, Zilbrysq helps reduce muscle fatigue and improve strength. Many patients report being able to perform daily tasks more comfortably and with less exhaustion.
Clinical studies have shown that patients using Zilbrysq experienced significant improvement in muscle function and symptom severity compared to placebo. Participants reported gains in mobility, speech, and breathing function, helping restore independence and confidence (FDA, 2023).
While Zilbrysq is not a cure for myasthenia gravis, it provides meaningful symptom control for many patients and may be used alongside other standard treatments, such as corticosteroids or immunosuppressants.
How does Zilbrysq work?
Zilbrysq works by blocking a part of the immune system called the complement system, which plays a central role in the autoimmune attack seen in generalized myasthenia gravis.
In people with AChR antibody-positive gMG, the immune system mistakenly produces antibodies that target the receptors on muscle cells. This attack activates the complement system, a series of proteins that further damage the junction between nerves and muscles, preventing proper communication and leading to weakness.
Zilbrysq specifically inhibits complement component 5 (C5), a protein that triggers this damaging process. By blocking C5, the medication stops the cascade of immune destruction, preserving the connection between nerves and muscles.
Clinically, this mechanism helps restore normal nerve signaling, allowing muscles to contract more effectively and reducing symptoms such as drooping eyelids, slurred speech, or difficulty swallowing. The targeted nature of Zilbrysq means it acts on a specific immune pathway rather than broadly suppressing the immune system, which may lower the risk of certain side effects associated with general immunosuppressive drugs.
Zilbrysq side effects
Like all prescription medicines, Zilbrysq can cause side effects. Most are mild and manageable, but some require close monitoring or medical attention.
Common side effects may include:
- Headache
- Diarrhea or stomach upset
- Injection site reactions (pain, redness, or swelling)
- Fever or mild fatigue
- Joint or muscle pain
Serious side effects (less common):
- Meningococcal infections (serious bacterial infections of the brain or bloodstream)
- Allergic or hypersensitivity reactions, including rash or difficulty breathing
- Upper respiratory infections
Because Zilbrysq weakens part of the immune system that helps fight certain bacteria, patients are at higher risk for meningococcal disease. To reduce this risk, vaccination against meningococcal infection is required before starting treatment, and boosters may be needed during therapy.
Patients should contact their doctor immediately if they experience symptoms such as fever, neck stiffness, rash, headache, or confusion possible signs of meningococcal infection.
Zilbrysq should be used with caution in patients who have active infections or are not up to date on their vaccines. Regular follow-up visits and lab monitoring help ensure treatment safety and effectiveness.
Zilbrysq dosage
Zilbrysq is a once-daily subcutaneous injection (abdomen, thigh, or upper arm) that most patients or caregivers can learn to self-inject at home after training. Doctors ensure meningococcal vaccines are administered and patients recognize infection symptoms before therapy.
During treatment, physicians monitor for infection, immune response, injection site reactions, and liver function/blood tests. Consistent daily dosing of Zilbrysq is crucial for effectiveness. Older adults and those with kidney or liver issues can use the same dosing but require close monitoring.
Does Zilbrysq have a generic version?
As of 2025, Zilbrysq (zilucoplan) does not have a generic version available in the United States or internationally. It is currently marketed exclusively under the UCB Pharma brand name. However, international versions may exist in other markets.
As a biologic, future alternatives will likely be biosimilars, requiring equivalent safety, strength, and effectiveness for FDA approval. Patients concerned about cost should discuss insurance, manufacturer programs, or financial assistance with their healthcare team; specialty pharmacies and patient-assistance programs can help.
Conclusion
Zilbrysq (zilucoplan) represents a major step forward in the treatment of acetylcholine receptor antibody-positive generalized myasthenia gravis. By precisely targeting the complement pathway, it helps prevent immune damage to nerve-muscle connections improving muscle strength, mobility, and quality of life for many adults with gMG.
While requiring daily injections and infection monitoring, Zilbrysq offers practical, effective long-term gMG management due to its self-administration and targeted mechanism. Success relies on patient-provider communication, dosing adherence, and prompt reporting of side effects or infection. For many, Zilbrysq provides renewed control, confidence, and hope for a more active life.
References
- U.S. Food and Drug Administration (FDA). (2023). FDA approves Zilbrysq (zilucoplan) for generalized myasthenia gravis. Retrieved from https://www.fda.gov
- Mayo Clinic. (2024). Zilucoplan injection: Uses and side effects. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Zilucoplan (subcutaneous route) drug information. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Complement inhibition in autoimmune neuromuscular disorders. Retrieved from https://www.nih.gov
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This study called Multicenter Retrospective Study on the Short- and Medium-Term Efficacy and Tolerance of Zilucoplan Therapy in a Cohort of French Patients with Anti-AChR Myasthenia Gravis, is investigating the effects of a new treatment called Zilucoplan (generic name: ZILBRYSQ) on patients in France with a condition known as myasthenia gravis. Myasthenia gravis is an autoimmune disease that caus...
Summary: The purpose of this study is to assess the steady state (SS) concentrations of zilucoplan (ZLP) and its major metabolites in mature breast milk of healthy study participants following injection of repeated once-daily doses of ZLP.
Summary: The purpose of this study is to assess the pharmacokinetics, pharmacodynamics, safety, tolerability, immunogenicity and activity of zilucoplan (ZLP) in pediatric study participants with generalized myasthenia gravis (gMG).
Related Latest Advances
Brand Information
- Complete or update vaccination for meningococcal bacteria (for serogroups A, C, W, Y, and B) at least 2 weeks prior to the first dose of ZILBRYSQ, unless the risks of delaying therapy outweigh the risk of developing a serious infection. Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for vaccination against meningococcal bacteria in patients receiving a complement inhibitor. See
- Patients receiving ZILBRYSQ are at increased risk for invasive disease caused by
- Serious Meningococcal Infections
- Other Infections
- Pancreatitis and Other Pancreatic Conditions

- Myasthenia Gravis Foundation of America (MGFA) clinical classification class II to IV,
- Positive serology for AChR binding autoantibodies,
- MG-Activities of Daily Living (MG-ADL) total score of ≥6,
- Those on MG therapy prior to screening (including acetylcholinesterase (AChE) inhibitors, steroids, or non-steroidal immunosuppressive therapies (NSISTs), either in combination or alone), needed to maintain a stable dose.
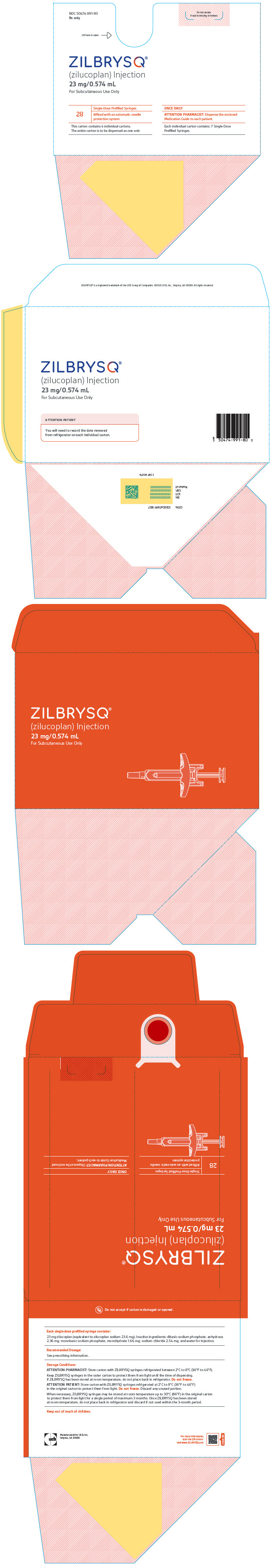
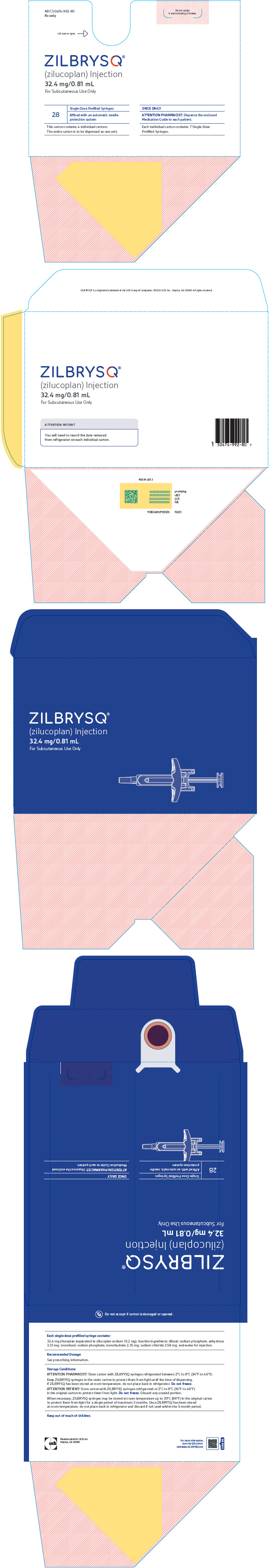
- Store ZILBRYSQ prefilled syringes in a refrigerator between 36°F to 46°F (2°C to 8°C) until the expiration date on the carton.
- ZILBRYSQ prefilled syringes may be stored at room temperature up to 86°F (30°C) in the original carton for a single period of up to 3 months. Write the date you removed the prefilled syringes from the refrigerator in the space provided on the carton. After ZILBRYSQ prefilled syringes have been stored at room temperature, do not place them back in the refrigerator. Throw them away if not used within 3 months or if the expiration date has passed, whichever occurs first.
- Keep the ZILBRYSQ prefilled syringes in the original carton before use.
- Keep ZILBRYSQ prefilled syringes and all medicines out of the reach of children.
- Do notuse ZILBRYSQ if the expiration date on the packaging has passed or the carton seals have been broken.
- Do notreuse the ZILBRYSQ prefilled syringe. The prefilled syringe is for 1-time (single use) only. You may get an infection.
- Do notinject ZILBRYSQ more than 1 time per day.
- Do notmiss any doses of ZILBRYSQ. If you miss your ZILBRYSQ dose, inject a dose as soon as possible. Then, inject the next dose at your scheduled time. Do not inject more than 1 dose each day.
- Do notuse the ZILBRYSQ prefilled syringe if it has been dropped.
- Do notremove the needle cap from the ZILBRYSQ prefilled syringe until you are ready to inject.
- Do notinsert the needle into the skin more than 1 time because this may bend or break the needle, causing trauma to the tissue.
- Do notpull back on the ZILBRYSQ prefilled syringe plunger head at any time because this can break the prefilled syringe.
- Do nottouch the needle guard activation clips at any time because this can cause the early activation of the needle guard.
- Take the ZILBRYSQ prefilled syringes carton out of the refrigerator and remove 1 prefilled syringe from the carton.
- Before you inject ZILBRYSQ, let the prefilled syringe warm up to room temperature on a clean flat surface
- Do not warm the ZILBRYSQ prefilled syringe in any other way (for example in a microwave, in hot water, or in direct sunlight).
- Do not remove the needle cap from the prefilled syringe until you are ready to inject.
- Remove 1 ZILBRYSQ prefilled syringe from the carton.
- Check the prefilled syringe for damage
- Do notuse if any part of the prefilled syringe appears to be cracked, leaking, or broken.
- Check thatthe needle cap is not cracked or broken and attached to the prefilled syringe (Figure C).
- Do notuse if the needle cap is missing or not securely attached.
- Check the expiration date and medicine name (ZILBRYSQ) on the prefilled syringe label
- Do notuse if the expiration date printed on the prefilled syringe has passed.
- Do notuse if the word ZILBRYSQ does not appear on the prefilled syringe.
- Check the dose appearing on the label
- Do notuse if the dose does not match your prescribed dose.
- Check the medicine inside the prefilled syringe
- Do notuse if the medicine is cloudy, discolored, or contains floating particles.
Figure C
- 1 ZILBRYSQ prefilled syringe
- 1 alcohol wipe (not supplied)
- 1 cotton ball or gauze pad (not supplied)
- 1 adhesive bandage (not supplied)
- 1 sharps disposal or puncture-resistant container (not supplied). See
- The stomach (abdomen), except for the 2-inch area around the belly button (navel)
- The front of the thighs
- The back of the upper arms (only if someone else is giving you the injection) (Figure E.2)
Figure E.2 – upper arms
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labelled to warn of hazardous waste inside the container.