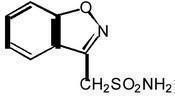
Zonisamide
What is Zonegran (Zonisamide)?
Top Global Experts
Related Clinical Trials
Summary: The purpose of this study is to develop population pharmacokinetic models for antiepileptic drugs in a pediatric population. The interest of these models is multiple: * describe the pharmacokinetics of these molecules in children and explain the inter-individual variability of concentrations through covariates such as weight, age, co-treatments, genetic polymorphisms and renal function; * estimate...
Summary: This study primarily aims to assess the efficacy and safety of zonisamide when used as an adjunctive therapy for focal epilepsy. The main questions it aims to answer are: 1. Does the frequency of epileptic seizures decrease after oral zonisamide, and does it improve cognitive function? 2. Are there any treatment-emergent adverse events associated with oral administration of zonisamide?
Summary: A phase II randomized, double-blind, placebo-controlled clinical trial (RCT) to evaluate the ability of zonisamide (ZON) to decrease alcohol use among treatment-seeking adults with an alcohol use disorder (AUD).
Related Latest Advances
Brand Information
- Serious skin rash that can cause death.
- Serious allergic reactions that may affect different parts of the body.
- Less sweating and increase in your body temperature (fever).
- Serious eye problems
- Suicidal thoughts or actions in some people.
- Increased level of acid in your blood (metabolic acidosis).
- Problems with your concentration, attention, memory, thinking, speech, or language.
- Blood cell changes such as reduced red and white blood cell counts.
- ZONEGRAN may cause a serious skin rash that can cause death. These serious skin reactions are more likely to happen when you begin taking ZONEGRAN within the first 4 months of treatment but may occur at later times.
- ZONEGRAN can cause other types of allergic reactions or serious problems that may affect different parts of the body such as your liver, kidneys, heart, or blood cells. You may or may not have a rash with these types of reactions. These reactions can be very serious and can cause death. Call your health care provider right away if you have:
- ZONEGRAN may cause you to sweat less and to increase your body temperature (fever). You may need to be hospitalized for this. You should watch for decreased sweating and fever, especially when it is hot and especially in children taking ZONEGRAN.
- ZONEGRAN may cause eye problems. Serious eye problems include:
- Like other antiepileptic drugs, ZONEGRAN may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
- ZONEGRAN can increase the level of acid in your blood (metabolic acidosis). If left untreated, metabolic acidosis can cause brittle or soft bones (osteoporosis, osteomalacia, osteopenia), kidney stones and can slow the rate of growth in children. Metabolic acidosis can happen with or without symptoms.
- ZONEGRAN may cause problems with your concentration, attention, memory, thinking, speech, or language.
- ZONEGRAN can cause blood cell changes such as reduced red and white blood cell counts. Call your healthcare provider if you develop fever, sore throat, sores in your mouth, or unusual bruising.
ZONEGRAN is a prescription medicine that is used with other medicines to treat partial seizures in adults.
It is not known if ZONEGRAN is safe or effective in children under 16 years of age.
Do not take ZONEGRAN:
Do not take ZONEGRAN if you are allergic to medicines that contain sulfa.
Before taking ZONEGRAN, tell your healthcare provider about all your medical conditions, including if you:
- have or have had depression, mood problems or suicidal thoughts or behavior
- have kidney problems
- have liver problems
- have a history of metabolic acidosis (too much acid in your blood)
- have weak, brittle bones or soft bones (osteomalacia, osteopenia or osteoporosis)
- have a growth problem
- are on a diet high in fat called a ketogenic diet
- have diarrhea
- have high blood levels of ammonia
- are pregnant or plan to become pregnant. ZONEGRAN may harm your unborn baby. Women who can become pregnant should use effective birth control. Tell your healthcare provider right away if you become pregnant while taking ZONEGRAN.
- are breastfeeding or plan to breastfeed. ZONEGRAN can pass into your breast milk. It is not known if ZONEGRAN in your breast milk can harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take ZONEGRAN.
- Take ZONEGRAN exactly as prescribed. Your healthcare prescriber may change your dose. Your healthcare provider will tell you how much ZONEGRAN to take.
- Take ZONEGRAN with or without food.
- Swallow the capsules whole.
- If you take too much ZONEGRAN, call your local Poison Control Center or go to the nearest emergency room right away.
- Do not stop taking ZONEGRAN without talking to your healthcare provider. Stopping ZONEGRAN suddenly can cause serious problems, including seizures that will not stop (status epilepticus).
- Do not drink alcohol or take other drugs that make you sleepy or dizzy while taking ZONEGRAN until you talk to your health care provider. ZONEGRAN taken with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how ZONEGRAN affects you. ZONEGRAN can slow your thinking and motor skills.
- kidney stones. Back pain, stomach pain, or blood in your urine may mean you have kidney stones. Drink plenty of fluids while you take ZONEGRAN to lower your chance of getting kidney stones.
- problems with mood or thinking (new or worse depression; sudden changes in mood, behavior, or loss of contact with reality, sometimes associated with hearing voices or seeing things that are not really there; feeling sleepy or tired; trouble concentrating; speech and language problems). Call your healthcare provider right away if you have any of the symptoms listed above.
- high blood ammonia levels. High ammonia in the blood can affect your mental activities, slow your alertness, make you feel tired, or cause vomiting.
- drowsiness
- loss of appetite
- dizziness
- problems with concentration or memory
- trouble with walking and coordination
- agitation or irritability
- Store ZONEGRAN between 59°F to 86°F (15°C to 30°C)
- Keep ZONEGRAN dry and away from light
ZONEGRAN® is a registered trademark of Mercury Pharma Group Limited in the United States and Puerto Rico.
Distributed by Advanz Pharma (US) Corp. under license.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
