Brand Name
Noxafil
Generic Name
Posaconazole
View Brand Information FDA approval date: September 15, 2006
Classification: Azole Antifungal
Form: Injection, Tablet, Powder, Suspension, Solution
What is Noxafil (Posaconazole)?
Posaconazole delayed-release tablets is an azole antifungal indicated as follows: Posaconazole delayed-release tablets are indicated for the prophylaxis of invasive Aspergillus and Candida infections in patients who are at high risk of developing these infections due to being severely immunocompromised, such as hematopoietic stem cell transplant recipients with graft-versus-host disease or those with hematologic malignancies with prolonged neutropenia from chemotherapy as follows.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
NOXAFIL (posaconazole)
1DOSAGE FORMS AND STRENGTHS
Noxafil injection
Noxafil injection (300 mg per vial) is available as a clear, colorless to yellow sterile liquid in a single-dose vial.
Noxafil Delayed-Release Tablets
Noxafil delayed-release tablets are available as yellow, coated, oblong tablets, debossed with "100" on one side containing 100 mg of posaconazole.
Noxafil Oral Suspension
Noxafil oral suspension is available as a white, cherry-flavored suspension in 4-ounce (123 mL) amber glass bottles with child-resistant closures containing 105 mL of suspension (40 mg of posaconazole per mL).
Noxafil PowderMix for Delayed-Release Oral Suspension
Each Noxafil PowderMix kit contains posaconazole, 300 mg, as an off-white to yellowish powder for delayed-release oral suspension and a mixing liquid.
2ADVERSE REACTIONS
The following serious and otherwise important adverse reactions are discussed in detail in another section of the labeling:
- Hypersensitivity
- Arrhythmias and QT Prolongation
- Hepatic Toxicity
2.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of Noxafil cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
2.2Postmarketing Experience
The following adverse reaction has been identified during the post-approval use of Noxafil. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency.
3DRUG INTERACTIONS
Posaconazole is primarily metabolized via UDP glucuronosyltransferase and is a substrate of p-glycoprotein (P-gp) efflux. Therefore, inhibitors or inducers of these clearance pathways may affect posaconazole plasma concentrations. Coadministration of drugs that can decrease the plasma concentrations of posaconazole should generally be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
Posaconazole is also a strong inhibitor of CYP3A4. Therefore, plasma concentrations of drugs predominantly metabolized by CYP3A4 may be increased by posaconazole
The following information was derived from data with Noxafil oral suspension or early tablet formulation unless otherwise noted. All drug interactions with Noxafil oral suspension, except for those that affect the absorption of posaconazole (via gastric pH and motility), are considered relevant to Noxafil injection, Noxafil delayed-release tablet, and Noxafil PowderMix for delayed-release oral suspension as well
3.1CYP3A4 Substrates
Concomitant administration of Noxafil with CYP3A4 substrates such as pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and cases of torsades de pointes. Therefore, Noxafil is contraindicated with these drugs
3.2HMG-CoA Reductase Inhibitors (Statins) Primarily Metabolized Through CYP3A4
Concomitant administration of Noxafil with simvastatin increases the simvastatin plasma concentrations by approximately 10-fold. Therefore, Noxafil is contraindicated with HMG-CoA reductase inhibitors primarily metabolized through CYP3A4
3.3Ergot Alkaloids
Most of the ergot alkaloids are substrates of CYP3A4. Noxafil may increase the plasma concentrations of ergot alkaloids (ergotamine and dihydroergotamine) which may lead to ergotism. Therefore, Noxafil is contraindicated with ergot alkaloids
3.4Benzodiazepines Metabolized by CYP3A4
Concomitant administration of Noxafil with midazolam increases the midazolam plasma concentrations by approximately 5-fold. Increased plasma midazolam concentrations could potentiate and prolong hypnotic and sedative effects. Concomitant use of Noxafil and other benzodiazepines metabolized by CYP3A4 (e.g., alprazolam, triazolam) could result in increased plasma concentrations of these benzodiazepines. Patients must be monitored closely for adverse effects associated with high plasma concentrations of benzodiazepines metabolized by CYP3A4 and benzodiazepine receptor antagonists must be available to reverse these effects
3.5Rifabutin
Rifabutin induces UDP-glucuronidase and decreases posaconazole plasma concentrations. Rifabutin is also metabolized by CYP3A4. Therefore, coadministration of rifabutin with Noxafil increases rifabutin plasma concentrations
3.6Phenytoin
Phenytoin induces UDP-glucuronidase and decreases posaconazole plasma concentrations. Phenytoin is also metabolized by CYP3A4. Therefore, coadministration of phenytoin with Noxafil increases phenytoin plasma concentrations
3.7Vinca Alkaloids
Most of the vinca alkaloids (e.g., vincristine and vinblastine) are substrates of CYP3A4. Concomitant administration of azole antifungals, including Noxafil, with vincristine has been associated with serious adverse reactions
3.8Calcium Channel Blockers Metabolized by CYP3A4
Noxafil may increase the plasma concentrations of calcium channel blockers metabolized by CYP3A4 (e.g., verapamil, diltiazem, nifedipine, nicardipine, felodipine). Frequent monitoring for adverse reactions and toxicity related to calcium channel blockers is recommended during coadministration. Dose reduction of calcium channel blockers may be needed.
3.9Digoxin
Increased plasma concentrations of digoxin have been reported in patients receiving digoxin and Noxafil. Therefore, monitoring of digoxin plasma concentrations is recommended during coadministration.
3.10Glipizide
Although no dosage adjustment of glipizide is required, it is recommended to monitor glucose concentrations when Noxafil and glipizide are concomitantly used.
3.11Alcohol
Posaconazole was found to release faster from Noxafil PowderMix for delayed-release oral suspension in the presence of alcohol
3.12Venetoclax
Concomitant use of venetoclax (a CYP3A4 substrate) with posaconazole increases venetoclax C
4OVERDOSAGE
There is no experience with overdosage of Noxafil injection and Noxafil delayed-release tablets.
During the clinical trials, some patients received Noxafil oral suspension up to 1600 mg/day with no adverse reactions noted that were different from the lower doses. In addition, accidental overdose was noted in one patient who took 1200 mg twice daily Noxafil oral suspension for 3 days. No related adverse reactions were noted by the investigator.
Posaconazole is not removed by hemodialysis.
5DESCRIPTION
Noxafil (posaconazole) is an azole antifungal agent. Noxafil is available as an injection solution to be diluted before intravenous administration, delayed-release tablet, oral suspension, and for delayed-release oral suspension intended for oral administration. Noxafil PowderMix for delayed-release oral suspension must be reconstituted before oral administration.
Posaconazole is designated chemically as 4-[4-[4-[4-[[ (3
Posaconazole is a white powder with a low aqueous solubility.
Noxafil Injection
Noxafil injection is available as a clear colorless to yellow, sterile liquid essentially free of foreign matter. Each vial contains 300 mg of posaconazole and the following inactive ingredients: 6.68 g Betadex Sulfobutyl Ether Sodium (SBECD), 0.0033 g edetate disodium, hydrochloric acid and sodium hydroxide to adjust the pH to 2.6, and water for injection.
Noxafil Delayed-Release Tablets
Noxafil delayed-release tablet is a yellow, coated, oblong tablet containing 100 mg of posaconazole. Each delayed-release tablet contains the inactive ingredients: croscarmellose sodium, hydroxypropylcellulose, hypromellose acetate succinate, iron oxide yellow, Macrogol/PEG 3350, magnesium stearate, microcrystalline cellulose, polyvinyl alcohol partially hydrolyzed, silicon dioxide, talc, and titanium dioxide.
Noxafil Oral Suspension
Noxafil oral suspension is a white, cherry-flavored immediate-release suspension containing 40 mg of posaconazole per mL and the following inactive ingredients: artificial cherry flavor, citric acid monohydrate, glycerin, liquid glucose, polysorbate 80, purified water, simethicone, sodium benzoate, sodium citrate dihydrate, titanium dioxide, and xanthan gum.
Noxafil PowderMix for Delayed-Release Oral Suspension
Noxafil PowderMix for delayed-release oral suspension is supplied as a component of a kit. Each kit contains Noxafil as an off-white to yellowish powder for delayed-release oral suspension, a bottle of mixing liquid, two 3 mL (green) notched tip syringes, two 10 mL (blue) notched tip syringes, two mixing cups, and one bottle adapter for the mixing liquid bottle. Noxafil PowderMix for delayed-release oral suspension contains 300 mg of posaconazole and the following inactive ingredient: hypromellose acetate succinate. The mixing liquid contains: anhydrous citric acid, antifoam Af emulsion, berry citrus sweet flavor, carboxymethylcellulose sodium, carrageenan calcium sulfate trisodium phosphate, glycerin, methylparaben, microcrystalline cellulose, potassium sorbate, propylparaben, purified water, sodium citrate, sodium phosphate monobasic monohydrate, sodium saccharin, sorbitol solution, and xanthan gum. Once reconstituted, the Noxafil PowderMix for delayed-release oral suspension will be cloudy and free of clumps.
6PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
Important Administration Instructions
Noxafil Delayed-Release Tablets
Advise patients that Noxafil delayed-release tablets must be swallowed whole and not divided, crushed, or chewed.
Instruct patients that if they miss a dose, they should take it as soon as they remember. If they do not remember until it is within 12 hours of the next dose, they should be instructed to skip the missed dose and go back to the regular schedule. Patients should not double their next dose or take more than the prescribed dose.
Noxafil Oral Suspension
Advise patients to take each dose of Noxafil oral suspension during or immediately (i.e., within 20 minutes) following a full meal. In patients who cannot eat a full meal, each dose of Noxafil oral suspension should be administered with a liquid nutritional supplement or an acidic carbonated beverage (e.g., ginger ale) in order to enhance absorption.
Instruct patients that if they miss a dose, they should take it as soon as they remember. However, if it is almost time for the next dose, they should be instructed to skip the missed dose and go back to the regular schedule. Patients should not double their next dose or take more than the prescribed dose.
Noxafil PowderMix for Delayed-Release Oral Suspension
Instruct parents and/or caregivers that ONLY the provided notched tip syringes can be used to administer Noxafil PowderMix for delayed-release oral suspension to pediatric patients.
Advise patients to take Noxafil PowderMix for delayed-release oral suspension with food.
Drug Interactions
Advise patients to inform their physician immediately if they:
- develop severe diarrhea or vomiting.
- are currently taking drugs that are known to prolong the QTc interval and are metabolized through CYP3A4.
- are currently taking a cyclosporine or tacrolimus, or they notice swelling in an arm or leg or shortness of breath.
- are taking other drugs or before they begin taking other drugs as certain drugs can decrease or increase the plasma concentrations of posaconazole.
Serious and Potentially Serious Adverse Reactions
Advise patients to inform their physician immediately if they:
- notice a change in heart rate or heart rhythm or have a heart condition or circulatory disease. Noxafil can be administered with caution to patients with potentially proarrhythmic conditions.
- are pregnant, plan to become pregnant, or are nursing.
- have liver disease or develop itching, nausea or vomiting, their eyes or skin turn yellow, they feel more tired than usual or feel like they have the flu.
- have ever had an allergic reaction to other antifungal medicines such as ketoconazole, fluconazole, itraconazole, or voriconazole.
Hereditary Fructose Intolerance (HFI)
Inform patients and caregivers that Noxafil PowderMix for delayed-release oral suspension contains sorbitol and can be life-threatening when administered to patients with hereditary fructose intolerance (HFI)
7PRINCIPAL DISPLAY PANEL - 105 mL Bottle Carton
NDC 0085-1328-01
NOXAFIL
(posaconazole)
(posaconazole)
Oral Suspension
200 mg/5 mL
Each mL contains: 40 mg posaconazole.
Attention: Noxafil Oral Suspension is
SHAKE WELL BEFORE EACH USE.
Take with a meal, or a nutritional supplement,
Carton contains measured dosing spoon.
Rx only

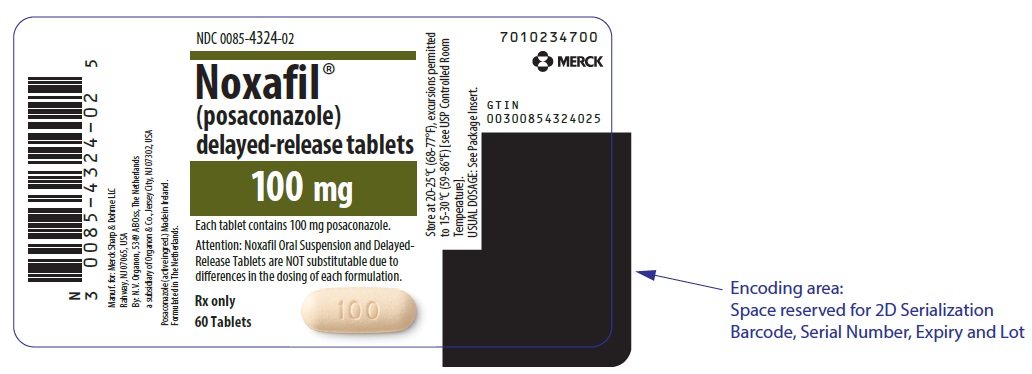
8PRINCIPAL DISPLAY PANEL - 60 Tablet Bottle Label
NDC 0085-4324-02
Noxafil
100 mg
Each tablet contains 100 mg posaconazole.
Attention: Noxafil Oral Suspension and Delayed-
Rx only
60 Tablets
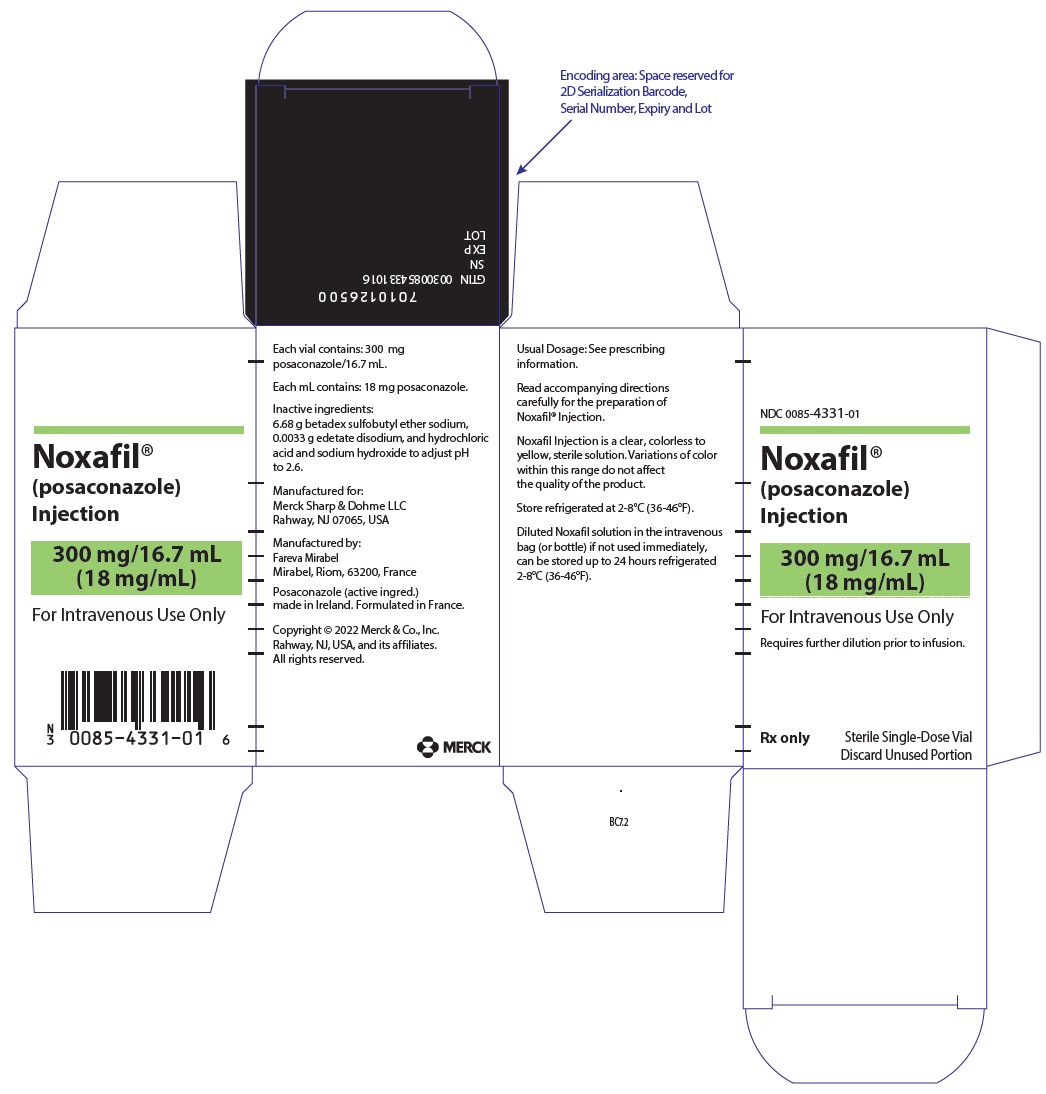
9PRINCIPAL DISPLAY PANEL - 16.7 mL Vial Carton
NDC 0085-4331-01
Noxafil
300 mg/16.7 mL
For Intravenous Use Only
Requires further dilution prior to infusion.
Rx only
Sterile Single-Dose Vial
Discard Unused Portion
Sterile Single-Dose Vial
Discard Unused Portion
10PRINCIPAL DISPLAY PANEL - 300 mg Carton
NDC 0085-2224-02
Noxafil
300 mg
For Pediatric Use
Each packet contains 300 mg posaconazole.
Attention: Noxafil PowderMix for Delayed-Release Oral Suspension is NOT
substitutable with Noxafil Oral Suspension due to differences in the
dosing of each formulation.
substitutable with Noxafil Oral Suspension due to differences in the
dosing of each formulation.
Use only the notched-tip syringes and mixing liquid provided with this kit.
Rx only
8 packets
8 packets
Multi-Dose Kit A
Additional notched-tip syringes are provided in
Package B with this kit.
DIRECTIONS FOR USE:
See instructions for use booklet and prescribing
information for additional information.
Additional notched-tip syringes are provided in
Package B with this kit.
DIRECTIONS FOR USE:
See instructions for use booklet and prescribing
information for additional information.
