Generic Name
Mupirocin
Brand Names
Pirnuo, DermacinRx Clorhexacin, Sx Pre-Op
FDA approval date: November 07, 2003
Classification: RNA Synthetase Inhibitor Antibacterial
Form: Ointment, Cream, Kit
What is Pirnuo (Mupirocin)?
Mupirocin Ointment USP, 2% is indicated for the topical treatment of impetigo due to susceptible isolates of Staphylococcus aureus and Streptococcus pyogenes . Mupirocin Ointment USP, 2% is an RNA synthetase inhibitor antibacterial indicated for the topical treatment of impetigo due to susceptible isolates of Staphylococcus aureus and Streptococcus pyogenes.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Pirnuo (Mupirocin)
1INDICATIONS AND USAGE
PIRNUO cream is indicated for the treatment of secondarily infected traumatic skin lesions (up to 10 cm in length or 100 cm
2DOSAGE AND ADMINISTRATION
- For Topical Use Only.
- Apply a small amount of PIRNUO cream, with a cotton swab or gauze pad, to the affected area 3 times daily for 10 days.
- Cover the treated area with gauze dressing if desired.
- Re-evaluate patients not showing a clinical response within 3 to 5 days.
- PIRNUO cream is not for intranasal, ophthalmic, or other mucosal use
- Do not apply PIRNUO cream concurrently with any other lotions, creams, or ointments
3DOSAGE FORMS AND STRENGTHS
PIRNUO
4CONTRAINDICATIONS
PIRNUO cream is contraindicated in patients with known hypersensitivity to mupirocin or any of the excipients of PIRNUO cream.
5ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Severe Allergic Reactions
- Eye Irritation
- Local Irritation
- Clostridium difficile-Associated Diarrhea [see Warnings and Precautions (
5.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In 2 randomized, double-blind, double-dummy trials, 339 subjects were treated with topical mupirocin cream plus oral placebo. Adverse reactions occurred in 28 (8.3%) subjects. The following adverse reactions were reported by at least 1% of subjects in connection with the use of mupirocin cream in clinical trials: headache (1.7%), rash (1.1%), and nausea (1.1%).
Other adverse reactions which occurred in less than 1% of subjects were: abdominal pain, burning at application site, cellulitis, dermatitis, dizziness, pruritus, secondary wound infection, and ulcerative stomatitis.
In a supportive trial in the treatment of secondarily infected eczema, 82 subjects were treated with mupirocin cream. The incidence of adverse reactions was as follows: nausea (4.9%), headache and burning at application site (3.6% each), pruritus (2.4%), and 1 report each of abdominal pain, bleeding secondary to eczema, pain secondary to eczema, hives, dry skin, and rash.
5.2Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following reactions have been identified during postmarketing use of mupirocin cream. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal relationship to mupirocin cream.
Immune System Disorders
Systemic allergic reactions, including anaphylaxis, urticaria, angioedema, and generalized rash
6DESCRIPTION
PIRNUO
The molecular formula of mupirocin calcium, USP is (C
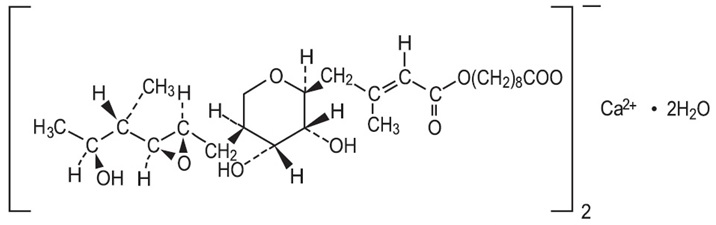
Figure 1. Structure of Mupirocin Calcium, USP
PIRNUO
7CLINICAL STUDIES
The efficacy of topical mupirocin cream for the treatment of secondarily infected traumatic skin lesions (e.g., lacerations, sutured wounds, and abrasions not more than 10 cm in length or 100 cm
Pediatrics
There were 93 pediatric subjects aged 2 weeks to 16 years enrolled per protocol in the secondarily infected skin lesion trials, although only 3 were younger than 2 years of age in the population treated with mupirocin cream. Subjects were randomized to either 10 days of topical mupirocin cream 3 times daily or 10 days of oral cephalexin (250 mg 4 times daily for subjects greater than 40 kg or 25 mg per kg per day oral suspension in 4 divided doses for subjects less than or equal to 40 kg). Clinical efficacy at follow-up (7 to 12 days post-therapy) in the per-protocol populations was 97.7% (43 of 44) for mupirocin cream and 93.9% (46 of 49) for cephalexin.
8REFERENCES
- Clinical and Laboratory Standards Institute (CLSI).
- Patel J, Gorwitz RJ, et al. Mupirocin Resistance.
- Clinical and Laboratory Standards Institute (CLSI).
- Clinical and Laboratory Standards Institute (CLSI).
- Finlay JE, Miller LA, Poupard JA. Interpretive criteria for testing susceptibility of staphylococci to mupirocin.
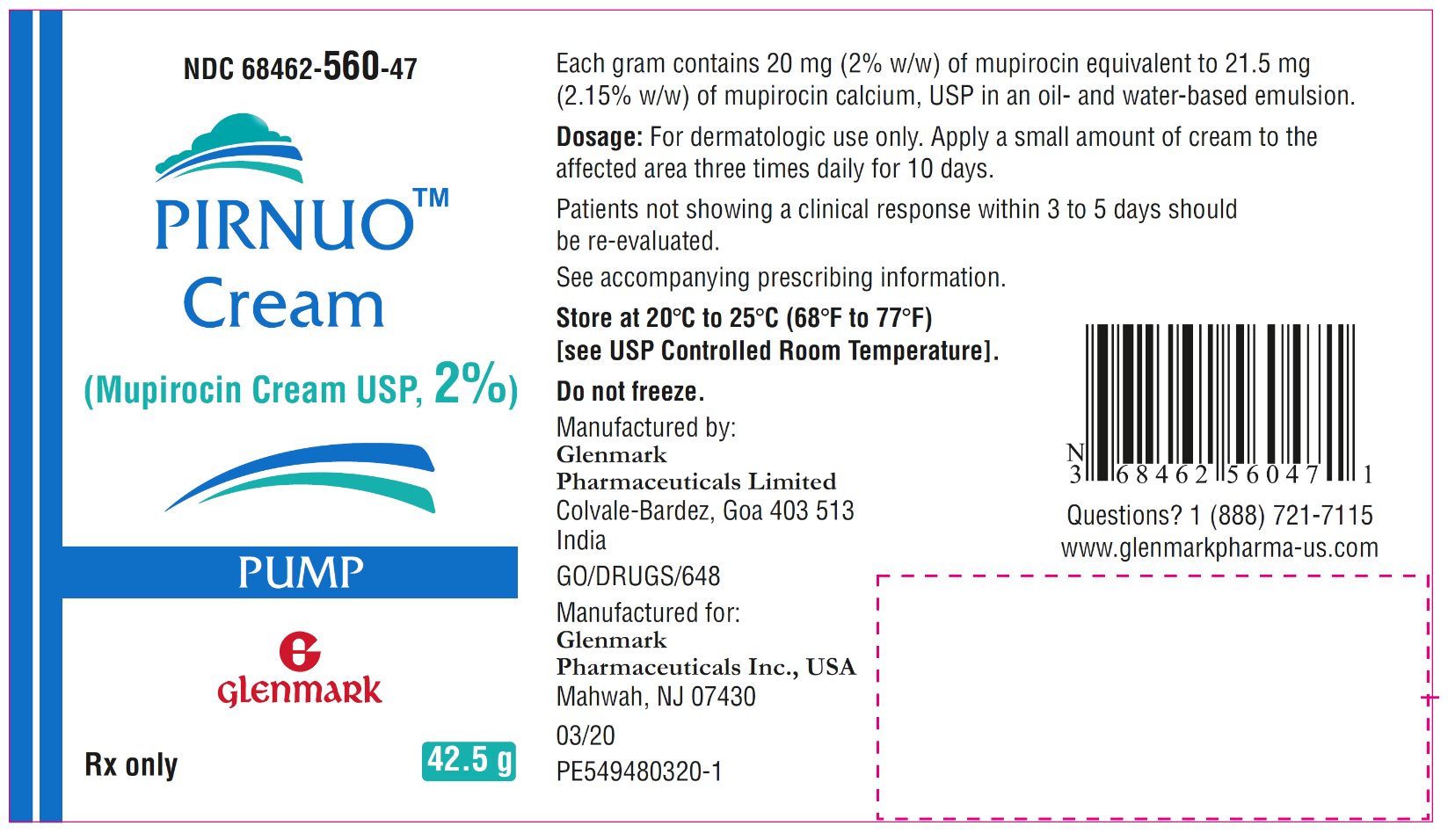
9HOW SUPPLIED/STORAGE AND HANDLING
PIRNUO
PIRNUO
- NDC 68462-560-47 42.5-gram pump (1 pump per carton)
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature]. Do not freeze.
10PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Advise the patient to administer PIRNUO cream as follows:
- Use PIRNUO cream only as directed by the healthcare provider. It is for external use only. Avoid contact of PIRNUO cream with the eyes. If PIRNUO cream gets in the eyes, rinse thoroughly with water.
- Do not use PIRNUO in cream in the nose.
- Wash your hands before and after applying PIRNUO cream.
- Use a gauze pad or cotton swab to apply a small amount of PIRNUO cream to the affected area. The treated area may be covered by gauze dressing if desired.
- Report to the healthcare provider any signs of local adverse reactions. PIRNUO cream should be stopped and the healthcare provider contacted if irritation, severe itching, or rash occurs.
- Report to the healthcare provider or go to the nearest emergency room if severe allergic reactions, such as swelling of the lips, face, or tongue, or wheezing occur
- If no improvement is seen in 3 to 5 days, contact the healthcare provider.
Trademarks are the property of their respective owners.
Manufactured by:
Glenmark Pharmaceuticals Limited
Colvale-Bardez, Goa - 403 513, India
Manufactured for:

Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888) 721-7115
www.glenmarkpharma-us.com
March 2020
PHARMACIST-DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT
11PATIENT INFORMATION
PIRNUO (Purr-NEW – oh)
(mupirocin calcium)
cream
What is PIRNUO cream?
PIRNUO cream is a prescription medicine used on the skin (topical use) to treat certain skin infections caused by bacteria called
Who should not use PIRNUO cream?
Do not use PIRNUO cream if:
- you are allergic to mupirocin or any of the ingredients in PIRNUO cream. See the end of this Patient Information leaflet for a complete list of the ingredients in PIRNUO cream.
What should I tell my healthcare provider before using PIRNUO cream?
Before using PIRNUO cream, tell your healthcare provider about all of your medical conditions including if you:
- are pregnant or plan to become pregnant. It is not known if PIRNUO cream will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if PIRNUO cream passes into your breast milk. You and your healthcare provider should decide if you can use PIRNUO cream while breastfeeding.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Do not mix PIRNUO cream with other lotions, creams, or ointments.
How should I use PIRNUO cream?
- PIRNUO cream is for use on the skin (topical). Do not get PIRNUO cream in your eyes, nose, mouth, or vagina (mucosal surfaces).
- Use PIRNUO cream exactly as your healthcare provider tells you to use it.
- Apply a small amount of PIRNUO cream, with a cotton swab or gauze pad, to the affected area 3 times each day. Apply PIRNUO cream for 10 days.
- It is important that you take the full course of PIRNUO cream. Do not stop early because your symptoms may disappear before the infection is fully cleared.
- Wash your hands
- After applying PIRNUO cream, you may cover the treated area with a clean gauze pad, unless your healthcare provider has told you to leave it uncovered.
- Talk to your healthcare provider if your skin does not improve after 3 to 5 days of treatment with PIRNUO cream.
- If you are breastfeeding and use PIRNUO cream on your breast or nipple, wash the area well before breastfeeding your child.
What are the possible side effects of PIRNUO cream?
PIRNUO cream may cause serious side effects, including:
- severe allergic reactions. Stop using PIRNUO cream and call your healthcare provider or go to the nearest emergency room right away if you have any of the following signs or symptoms of a severe allergic reaction:
- eye irritation. Do not get PIRNUO cream in your eyes. If PIRNUO cream gets in your eyes, rinse your eyes well with water.
- irritation in the area PIRNUO cream is used. A rash may occur after using PIRNUO cream and can be severe. Stop using PIRNUO cream and call your healthcare provider if you develop an irritation, severe itching, or a rash while using PIRNUO cream.
- a type of diarrhea called CDAD may happen in people who use or have used medicine to treat bacterial infections. The severity of CDAD can range from mild diarrhea to severe diarrhea that may cause death (fatal colitis). Call your healthcare provider or go to the nearest emergency room right away if you have diarrhea while using or after you stop using PIRNUO cream.
The most common side effects of PIRNUO cream include:
- headache
- rash
- nausea
These are not all the possible side effects of PIRNUO cream. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store PIRNUO cream?
- Store PIRNUO cream at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze PIRNUO cream.
- Keep PIRNUO cream and all medicines out of the reach of children.
General information about the safe and effective use of PIRNUO cream.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use PIRNUO cream for a condition for which it was not prescribed. Do not give PIRNUO cream to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about PIRNUO cream that is written for health professionals.
What are the ingredients in PIRNUO cream?
Active Ingredient: mupirocin calcium
Inactive Ingredients: benzyl alcohol, glycerol monostearate, mineral oil, phenoxyethanol, polyoxyl 20 cetostearyl ether, purified water and xanthan gum.
Trademarks are the property of their respective owners.
Manufactured by:
Glenmark Pharmaceuticals Limited
Colvale-Bardez, Goa - 403 513, India
Manufactured for:

Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888)721-7115
www.glenmarkpharma-us.com
- This Patient Information has been approved by the U.S. Food and Drug Administration Revised: March 2020
12Package/Label Display Panel
NDC-68462-560-47
PIRNUO Cream
42.5 – Carton – Pump Pack

13Package/Label Display Panel
NDC-68462-560-47
PIRNUO Cream
42.5 – Container Label – Pump Pack