Brand Name
Zioptan
Generic Name
Tafluprost
View Brand Information FDA approval date: February 08, 2022
Classification: Prostaglandin Analog
Form: Solution
What is Zioptan (Tafluprost)?
Tafluprost Ophthalmic Solution.
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Inflammatory Biomarkers in Ocular Surface in Patients With Primary Open Angle Glaucoma and Ocular Hypertension Under Topical Prostaglandin Therapy
Summary: Glaucoma is a chronic optic neuropathy whose main modifiable risk factor is an abnormally elevated intraocular pressure. The aim of glaucoma treatment is to slow the progression of the disease by reducing intraocular pressure. Prostaglandin derivatives are the most effective topical drugs in reducing intraocular pressure (IOP). Among these, latanoprost was the first agent of this type to be approv...
Related Latest Advances
Brand Information
ZIOPTAN (Tafluprost)
1INDICATIONS AND USAGE
ZIOPTAN
2DOSAGE AND ADMINISTRATION
The recommended dose is one drop of ZIOPTAN
The dose should not exceed once daily since it has been shown that more frequent administration of prostaglandin analogs may lessen the intraocular pressure lowering effect.
Reduction of the intraocular pressure starts approximately 2 to 4 hours after the first administration with the maximum effect reached after 12 hours.
ZIOPTAN
The solution from one individual unit is to be used immediately after opening for administration to one or both eyes. Since sterility cannot be maintained after the individual unit is opened, the remaining contents should be discarded immediately after administration.
3DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing tafluprost 0.015 mg/mL.
4CONTRAINDICATIONS
None.
5DESCRIPTION
Tafluprost is a fluorinated analog of prostaglandin F2α. The chemical name for tafluprost is 1-methylethyl (5Z)-7-{(1R,2R,3R,5S)-2-[(1E)-3,3-difluoro-4-phenoxy-1-butenyl}-3,5-dihydroxycyclopen t y l]-5-heptenoate. The molecular formula of tafluprost is C
Its structural formula is:
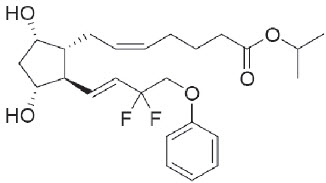
Tafluprost is a colorless to light yellow viscous liquid that is practically insoluble in water.
ZIOPTAN
ZIOPTAN
ZIOPTAN
6CLINICAL STUDIES
In clinical studies up to 24 months in duration, patients with open-angle glaucoma or ocular hypertension and baseline pressure of 23 to 26 mmHg who were treated with ZIOPTAN
7HOW SUPPLIED/STORAGE AND HANDLING
ZIOPTAN
NDC 82584-609-30; Unit-of-Use Carton of 30.
8PATIENT COUNSELING INFORMATION
See
8.1Nightly Application
Advise patients to not exceed once daily dosing since more frequent administration may decrease the intraocular pressure lowering effect of ZIOPTAN
8.2Handling the Single-Use Container
Advise patients that ZIOPTAN
8.3Potential for Pigmentation
Advise patients about the potential for increased brown pigmentation of the iris, which may be permanent. Also inform patients about the possibility of eyelid skin darkening, which may be reversible after discontinuation of ZIOPTAN
8.4Potential for Eyelash Changes
Inform patients of the possibility of eyelash and vellus hair changes in the treated eye during treatment with ZIOPTAN
8.5When to Seek Physician Advice
Advise patients that if they develop a new ocular condition (e.g., trauma or infection), experience a sudden decrease in visual acuity, have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician's advice concerning the continued use of ZIOPTAN
8.6Use with Other Ophthalmic Drugs
If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes between applications.
8.7Storage Information
Instruct patients on proper storage of cartons, unopened foil pouches, and opened foil pouches
9PATIENT INFORMATION
ZIOPTAN (zye OP tan)
(tafluprost ophthalmic solution) 0.0015%
(tafluprost ophthalmic solution) 0.0015%
Read this Patient Information before you start using ZIOPTAN
What is ZIOPTAN
ZIOPTAN
ZIOPTAN
What should I tell my doctor before using ZIOPTAN®?
Before you use ZIOPTAN
- have or have had eye problems including any surgery on your eye or eyes
- are using any other eye medicines
- have any other medical problems
- are pregnant or plan to become pregnant. It is not known if ZIOPTAN
- are breastfeeding or plan to breastfeed. It is not known if ZIOPTAN
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take ZIOPTAN®?
Read the Instructions for Use at the end of this Patient Information leaflet for additional instructions about the right way to use ZIOPTAN
- Use 1 drop of ZIOPTAN Talk to your doctor or pharmacist if you are not sure how to use ZIOPTAN ®.
- Your ZIOPTAN
- If you use other medicines in your eye, wait at least 5 minutes between using ZIOPTAN
- Use your ZIOPTAN
What are the possible side effects of ZIOPTAN
ZIOPTAN
- changes in the color of your eye (iris). Your iris may become more brown in color while using ZIOPTAN ®. This color change may not go away when you stop using ZIOPTAN ®. If ZIOPTAN ® is used in 1 eye only, the color of that eye may always be a different color from the color of your other eye.
- darkening of the color of the skin around your eye (eyelid). These skin changes usually go away when you stop using ZIOPTAN ®.
- increasing the length, thickness, color, or number of your eyelashes. These eyelash changes usually go away when you stop using ZIOPTAN ®.
- hair growth on your eyelids. This hair growth usually goes away when you stop using ZIOPTAN ®.
The most common side effects of ZIOPTAN
- redness, stinging or itching of your eye
- cataract formation
- dry eye
- eye pain
- blurred vision
- headache
- common cold
- cough
- urinary tract infection
Tell your doctor if you have any new eye problems while using ZIOPTAN
- an eye injury
- an eye infection
- a sudden loss of vision
- eye surgery
- swelling and redness of and around your eye (conjunctivitis)
- problems with your eyelids
Additionally, the following side effects have been reported in general use:
- worsening of asthma
- shortness of breath
Tell your doctor if you have any other side effects that bother you.
These are not all the possible side effects of ZIOPTAN
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ZIOPTAN
Important information for Mail-Order Patients:
Do not use if prescription is not received within two days of dispensing date.
Keep the foil pouches and ZIOPTAN
Before opening the foil pouches:
- Store the unopened foil pouches in a refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not open the pouch containing ZIOPTAN
After opening the foil pouch:
- Store the opened foil pouch at room temperature, between 68°F to 77°F (20°C to 25°C), for up to 30 days.
- Throw away all unused ZIOPTAN
- Keep the ZIOPTAN
- After opening the foil pouch, refrigeration is not required.
Keep ZIOPTAN
General information about the safe and effective use of ZIOPTAN
Do not use ZIOPTAN
This Patient Information leaflet summarizes the most important information about ZIOPTAN
What are the ingredients in ZIOPTAN
Active ingredients: tafluprost
Inactive ingredients: glycerol, sodium dihydrogen phosphate dihydrate, disodium edetate, and polysorbate 80, hydrochloric acid and/or sodium hydroxide, and water for injection.
10Instructions for Use
Read these Instructions for Use before using your ZIOPTAN
Important:
- ZIOPTAN
- ZIOPTAN
- Do not use the ZIOPTAN ® single-use containers if the foil pouch is opened.
- Write down the date you open the foil pouch in the space provided on the pouch.
Every time you use ZIOPTAN
- If your doctor has told you to use ZIOPTAN
- There is enough ZIOPTAN
- Throw away the opened single-use container with any remaining ZIOPTAN® right away.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Rx only
Manufactured for :
Waltham, MA 02451
Made in France
Revised: 05/2023
N10260U30USA/0423
Revised: 3/2024
Thea Pharma Inc.
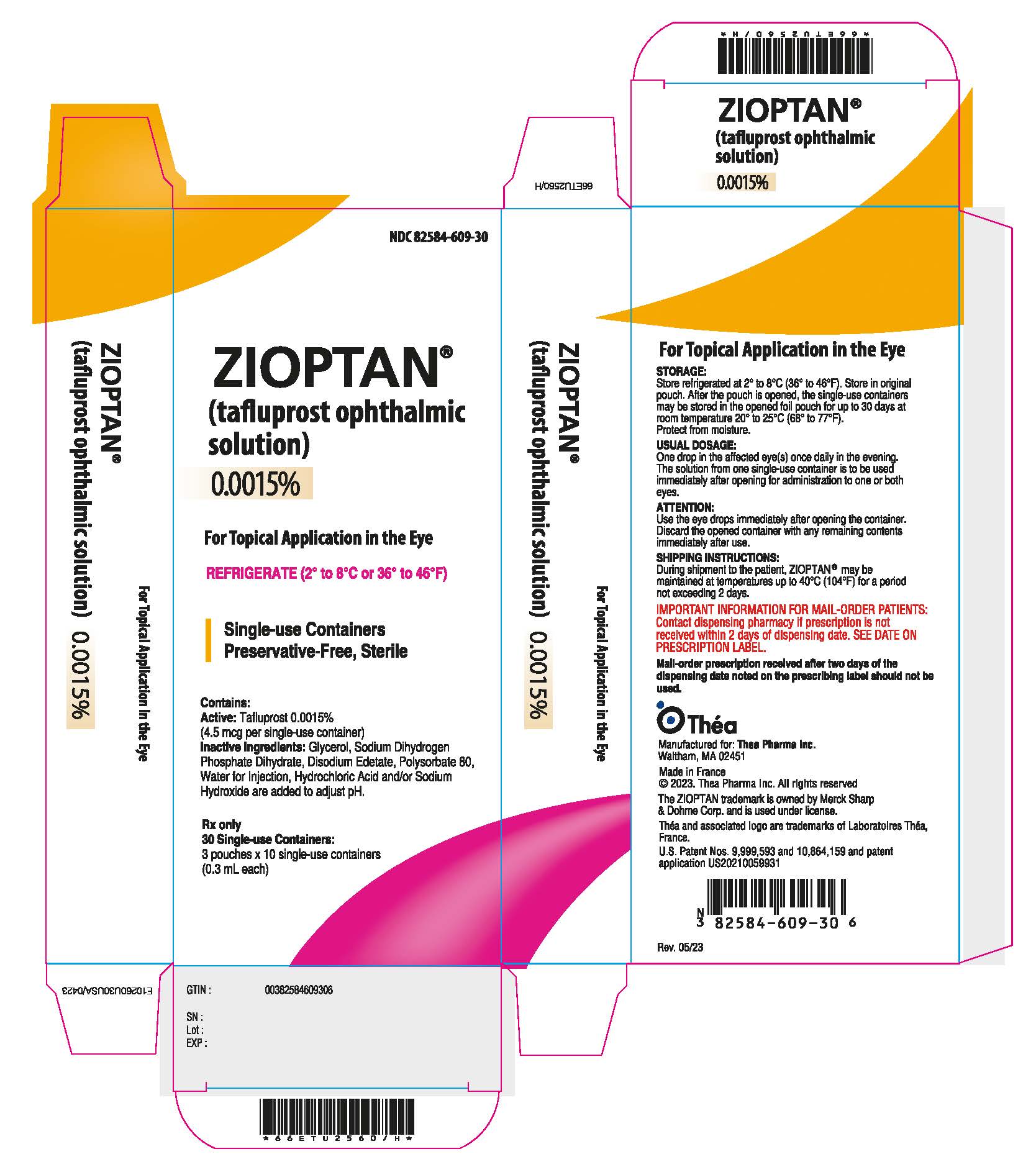
11PRINCIPAL DISPLAY PANEL - 0.3 mL Container Pouch Carton
NDC 82584-609-30
ZIOPTAN ®
For Topical Application in the Eye
REFRIGERATE (2° to 8°C or 36° to 46°F)
Single-use Containers
Contains:
Rx only