Brand Name
Diflucan
Generic Name
Fluconazole
View Brand Information FDA approval date: December 23, 1993
Classification: Azole Antifungal
Form: Injection, Tablet, Powder
What is Diflucan (Fluconazole)?
Fluconazole Injection, USP is indicated for the treatment of: Oropharyngeal and esophageal candidiasis. In open noncomparative studies of relatively small numbers of patients, fluconazole was also effective for the treatment of Candida urinary tract infections, peritonitis, and systemic Candida infections including candidemia, disseminated candidiasis, and pneumonia. Cryptococcal meningitis. Before prescribing fluconazole for AIDS patients with cryptococcal meningitis, please see CLINICAL STUDIES section. Studies comparing fluconazole to amphotericin B in non-HIV infected patients have not been conducted. Prophylaxis: Fluconazole Injection, USP is also indicated to decrease the incidence of candidiasis in patients undergoing bone marrow transplantation who receive cytotoxic chemotherapy and/or radiation therapy. Specimens for fungal culture and other relevant laboratory studies should be obtained prior to therapy to isolate and identify causative organisms. Therapy may be instituted before the results of the cultures and other laboratory studies are known; however, once these results become available, anti-infective therapy should be adjusted accordingly.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Diflucan (fluconazole)
1DESCRIPTION
DIFLUCAN
Fluconazole is designated chemically as 2,4-difluoro-α,α

Fluconazole is a white crystalline solid which is slightly soluble in water and saline.
DIFLUCAN Tablets contain 50 mg, 100 mg, 150 mg, or 200 mg of fluconazole and the following inactive ingredients: microcrystalline cellulose, dibasic calcium phosphate anhydrous, povidone, croscarmellose sodium, FD&C Red No. 40 aluminum lake dye, and magnesium stearate.
DIFLUCAN for Oral Suspension contains 350 mg or 1400 mg of fluconazole and the following inactive ingredients: sucrose, sodium citrate dihydrate, citric acid anhydrous, sodium benzoate, titanium dioxide, colloidal silicon dioxide, xanthan gum, and natural orange flavor. After reconstitution with 24 mL of distilled water or Purified Water (USP), each mL of reconstituted suspension contains 10 mg or 40 mg of fluconazole.
2INDICATIONS AND USAGE
DIFLUCAN (fluconazole) is indicated for the treatment of:
- Vaginal candidiasis (vaginal yeast infections due to
- Oropharyngeal and esophageal candidiasis. In open noncomparative studies of relatively small numbers of patients, DIFLUCAN was also effective for the treatment of
- Cryptococcal meningitis. Before prescribing DIFLUCAN (fluconazole) for AIDS patients with cryptococcal meningitis, please see
2.1Prophylaxis:
DIFLUCAN is also indicated to decrease the incidence of candidiasis in patients undergoing bone marrow transplantation who receive cytotoxic chemotherapy and/or radiation therapy.
Specimens for fungal culture and other relevant laboratory studies (serology, histopathology) should be obtained prior to therapy to isolate and identify causative organisms. Therapy may be instituted before the results of the cultures and other laboratory studies are known; however, once these results become available, anti-infective therapy should be adjusted accordingly.
3CONTRAINDICATIONS
DIFLUCAN (fluconazole) is contraindicated in patients who have shown hypersensitivity to fluconazole or to any of its excipients. There is no information regarding cross-hypersensitivity between fluconazole and other azole antifungal agents. Caution should be used in prescribing DIFLUCAN to patients with hypersensitivity to other azoles. Coadministration of other drugs known to prolong the QT interval and which are metabolized via the enzyme CYP3A4 such as erythromycin, pimozide, and quinidine are contraindicated in patients receiving fluconazole. (See
4WARNINGS
(1) Hepatic injury: DIFLUCAN should be administered with caution to patients with liver dysfunction. DIFLUCAN has been associated with rare cases of serious hepatic toxicity, including fatalities primarily in patients with serious underlying medical conditions. In cases of DIFLUCAN-associated hepatotoxicity, no obvious relationship to total daily dose, duration of therapy, sex, or age of the patient has been observed. DIFLUCAN hepatotoxicity has usually, but not always, been reversible on discontinuation of therapy. Patients who develop abnormal liver function tests during DIFLUCAN therapy should be monitored for the development of more severe hepatic injury. DIFLUCAN should be discontinued if clinical signs and symptoms consistent with liver disease develop that may be attributable to DIFLUCAN.
(2) Anaphylaxis: In rare cases, anaphylaxis has been reported.
(3) Dermatologic: Exfoliative skin disorders during treatment with DIFLUCAN have been reported. Fatal outcomes have been reported in patients with serious underlying diseases. Patients with deep seated fungal infections who develop rashes during treatment with DIFLUCAN should be monitored closely and the drug discontinued if lesions progress. Fluconazole should be discontinued in patients treated for superficial fungal infection who develop a rash that may be attributed to fluconazole.
(4) Potential for fetal harm: There are no adequate and well-controlled clinical trials of DIFLUCAN in pregnant women. Case reports describe a pattern of distinct congenital anomalies in infants exposed
5ADVERSE REACTIONS
DIFLUCAN is generally well tolerated.
In some patients, particularly those with serious underlying diseases such as AIDS and cancer, changes in renal and hematological function test results and hepatic abnormalities have been observed during treatment with fluconazole and comparative agents, but the clinical significance and relationship to treatment is uncertain.
5.1In Patients Receiving a Single Dose for Vaginal Candidiasis:
During comparative clinical studies conducted in the United States, 448 patients with vaginal candidiasis were treated with DIFLUCAN, 150 mg single dose. The overall incidence of side effects possibly related to DIFLUCAN was 26%. In 422 patients receiving active comparative agents, the incidence was 16%. The most common treatment-related adverse events reported in the patients who received 150 mg single dose fluconazole for vaginitis were headache (13%), nausea (7%), and abdominal pain (6%). Other side effects reported with an incidence equal to or greater than 1% included diarrhea (3%), dyspepsia (1%), dizziness (1%), and taste perversion (1%). Most of the reported side effects were mild to moderate in severity. Rarely, angioedema and anaphylactic reaction have been reported in marketing experience.
5.2In Patients Receiving Multiple Doses for Other Infections:
Sixteen percent of over 4000 patients treated with DIFLUCAN (fluconazole) in clinical trials of 7 days or more experienced adverse events. Treatment was discontinued in 1.5% of patients due to adverse clinical events and in 1.3% of patients due to laboratory test abnormalities.
Clinical adverse events were reported more frequently in HIV infected patients (21%) than in non-HIV infected patients (13%); however, the patterns in HIV infected and non-HIV infected patients were similar. The proportions of patients discontinuing therapy due to clinical adverse events were similar in the two groups (1.5%).
The following treatment-related clinical adverse events occurred at an incidence of 1% or greater in 4048 patients receiving DIFLUCAN for 7 or more days in clinical trials: nausea 3.7%, headache 1.9%, skin rash 1.8%, vomiting 1.7%, abdominal pain 1.7%, and diarrhea 1.5%.
5.3Hepato-biliary:
In combined clinical trials and marketing experience, there have been rare cases of serious hepatic reactions during treatment with DIFLUCAN. (See
In two comparative trials evaluating the efficacy of DIFLUCAN for the suppression of relapse of cryptococcal meningitis, a statistically significant increase was observed in median AST (SGOT) levels from a baseline value of 30 IU/L to 41 IU/L in one trial and 34 IU/L to 66 IU/L in the other. The overall rate of serum transaminase elevations of more than 8 times the upper limit of normal was approximately 1% in fluconazole-treated patients in clinical trials. These elevations occurred in patients with severe underlying disease, predominantly AIDS or malignancies, most of whom were receiving multiple concomitant medications, including many known to be hepatotoxic. The incidence of abnormally elevated serum transaminases was greater in patients taking DIFLUCAN concomitantly with one or more of the following medications: rifampin, phenytoin, isoniazid, valproic acid, or oral sulfonylurea hypoglycemic agents.
5.4Post-Marketing Experience
In addition, the following adverse events have occurred during post-marketing experience.
Immunologic: In rare cases, anaphylaxis (including angioedema, face edema and pruritus) has been reported.
Body as a Whole: Asthenia, fatigue, fever, malaise.
Central Nervous System: Seizures, dizziness.
Hematopoietic and Lymphatic: Leukopenia, including neutropenia and agranulocytosis, thrombocytopenia.
Metabolic: Hypercholesterolemia, hypertriglyceridemia, hypokalemia.
Gastrointestinal: Cholestasis, dry mouth, hepatocellular damage, dyspepsia, vomiting.
Other Senses: Taste perversion.
Musculoskeletal System: myalgia.
Nervous System: Insomnia, paresthesia, somnolence, tremor, vertigo.
Skin and Appendages: Acute generalized exanthematous pustulosis, drug eruption including fixed drug eruption, increased sweating, exfoliative skin disorders including Stevens-Johnson syndrome and toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS) (See WARNINGS), alopecia.
5.5Adverse Reactions in Pediatric Patients:
The pattern and incidence of adverse events and laboratory abnormalities recorded during pediatric clinical trials are comparable to those seen in adults.
In Phase II/III clinical trials conducted in the United States and in Europe, 577 pediatric patients, ages 1 day to 17 years were treated with DIFLUCAN at doses up to 15 mg/kg/day for up to 1,616 days. Thirteen percent of pediatric patients experienced treatment-related adverse events. The most commonly reported events were vomiting (5%), abdominal pain (3%), nausea (2%), and diarrhea (2%). Treatment was discontinued in 2.3% of patients due to adverse clinical events and in 1.4% of patients due to laboratory test abnormalities. The majority of treatment-related laboratory abnormalities were elevations of transaminases or alkaline phosphatase.
Clinical Trials Experience in Pediatric Patients
Safety in Prophylaxis of Invasive
In a Phase 3 clinical trial of pediatric patients (premature infants weighing less than 750 grams at birth), the incidence of intestinal perforation in infants receiving DIFLUCAN prophylaxis was higher compared to infants receiving placebo (see
Safety in Pediatric Patients Receiving ECMO
A cohort of 20 pediatric patients (1 day to 17 years of age) on ECMO received DIFLUCAN in a prospective, open-label, single-center safety and PK ECMO study. The adverse reaction profile of DIFLUCAN in these patients was similar to that of adult and pediatric non-ECMO patients (See
6OVERDOSAGE
There have been reports of overdose with fluconazole accompanied by hallucination and paranoid behavior.
In the event of overdose, symptomatic treatment (with supportive measures and gastric lavage if clinically indicated) should be instituted.
Fluconazole is largely excreted in urine. A 3-hour hemodialysis session decreases plasma levels by approximately 50%.
In mice and rats receiving very high doses of fluconazole, clinical effects in both species included decreased motility and respiration, ptosis, lacrimation, salivation, urinary incontinence, loss of righting reflex, and cyanosis; death was sometimes preceded by clonic convulsions.
7HOW SUPPLIED
DIFLUCAN Tablets: Pink trapezoidal tablets containing 50, 100, or 200 mg of fluconazole are packaged in bottles or unit dose blisters. The 150 mg fluconazole tablets are pink and oval shaped, packaged in a single dose unit blister.
DIFLUCAN Tablets are supplied as follows:
DIFLUCAN 50 mg Tablets: Engraved with "DIFLUCAN" and "50" on the front and "ROERIG" on the back.
DIFLUCAN 100 mg Tablets: Engraved with "DIFLUCAN" and "100" on the front and "ROERIG" on the back.
DIFLUCAN 150 mg Tablets: Engraved with "DIFLUCAN" and "150" on the front and "ROERIG" on the back.
DIFLUCAN 200 mg Tablets: Engraved with "DIFLUCAN" and "200" on the front and "ROERIG" on the back.
7.1Storage
Store tablets below 30°C (86°F).
DIFLUCAN for Oral Suspension: DIFLUCAN for Oral Suspension is supplied as an orange-flavored powder to provide 35 mL per bottle as follows:
7.2Storage:
Store dry powder below 30°C (86°F). Store reconstituted suspension between 30°C (86°F) and 5°C (41°F) and discard unused portion after 2 weeks. Protect from freezing.
8PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle Label
Pfizer
NDC 0049-3410-30
DIFLUCAN
(Fluconazole Tablets)
(Fluconazole Tablets)
50 mg
30 Tablets

9PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Label
Pfizer
NDC 0049-3420-30
DIFLUCAN
(Fluconazole Tablets)
(Fluconazole Tablets)
100 mg
30 Tablets

10PRINCIPAL DISPLAY PANEL - 100 mg Tablet Blister Pack
DIFLUCAN®
(Fluconazole Tablet)
(Fluconazole Tablet)
100 mg
Distributed by
13041200
EXP & LOT AREA
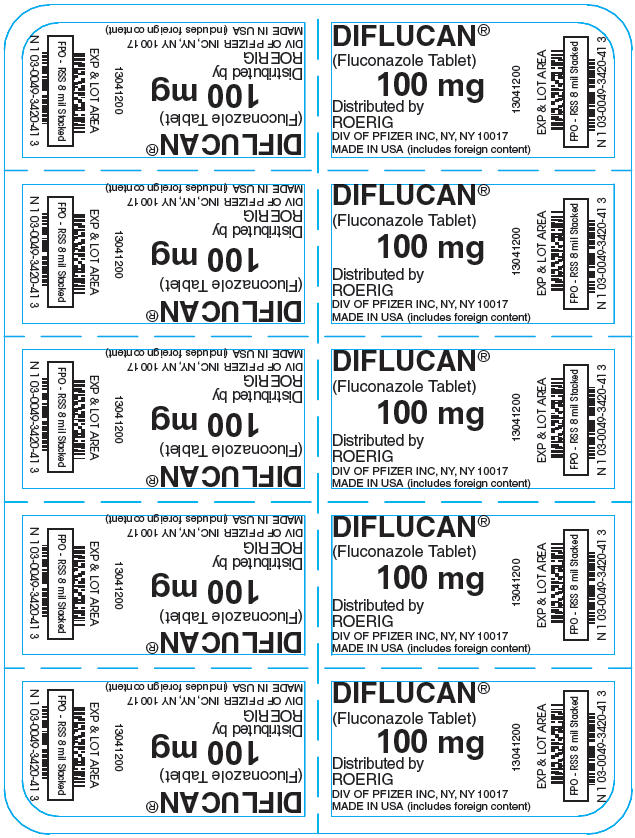
11PRINCIPAL DISPLAY PANEL - 100 mg Tablet Blister Pack Carton
UNIT DOSE
Pfizer
NDC 0049-3420-41
DIFLUCAN®
(Fluconazole Tablets)
(Fluconazole Tablets)
100 mg
For in-institution use only
100 Tablets

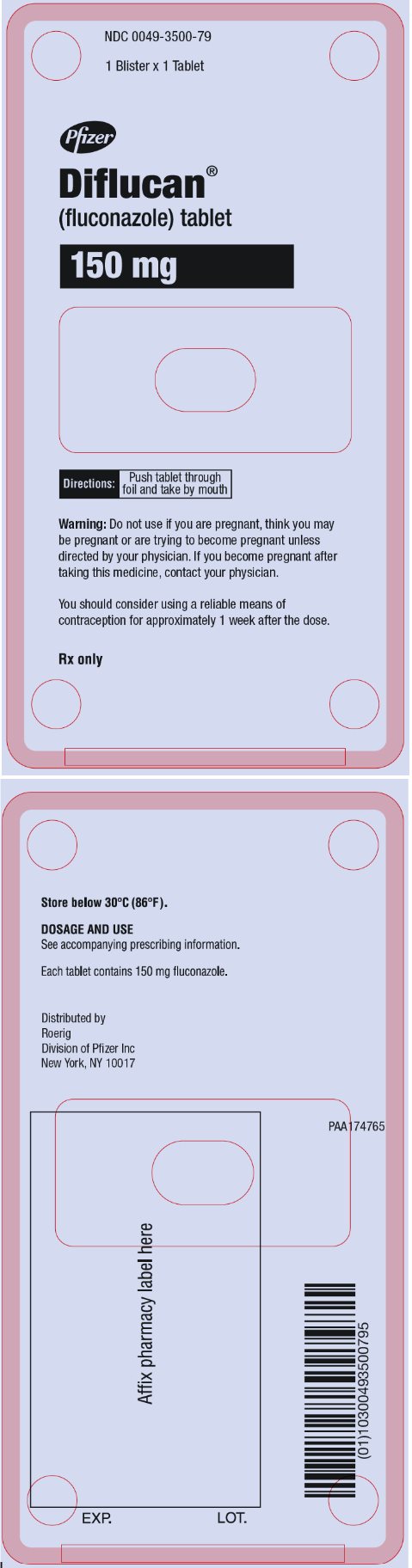
12PRINCIPAL DISPLAY PANEL - 150 mg Tablet Blister Pack
NDC 0049-3500-79
1 Blister x 1 Tablet
Pfizer
Diflucan®
(fluconazole tablet)
150 mg
(fluconazole tablet)
150 mg
Directions:
Push tablet through
foil and take by mouth
Push tablet through
foil and take by mouth
Warning: Do not use if you are pregnant, think you may
be pregnant or are trying to become pregnant unless
directed by your physician. If you become pregnant after
taking this medicine, contact your physician.
You should consider using a reliable means of
contraception for approximately 1 week after the dose.
Rx only
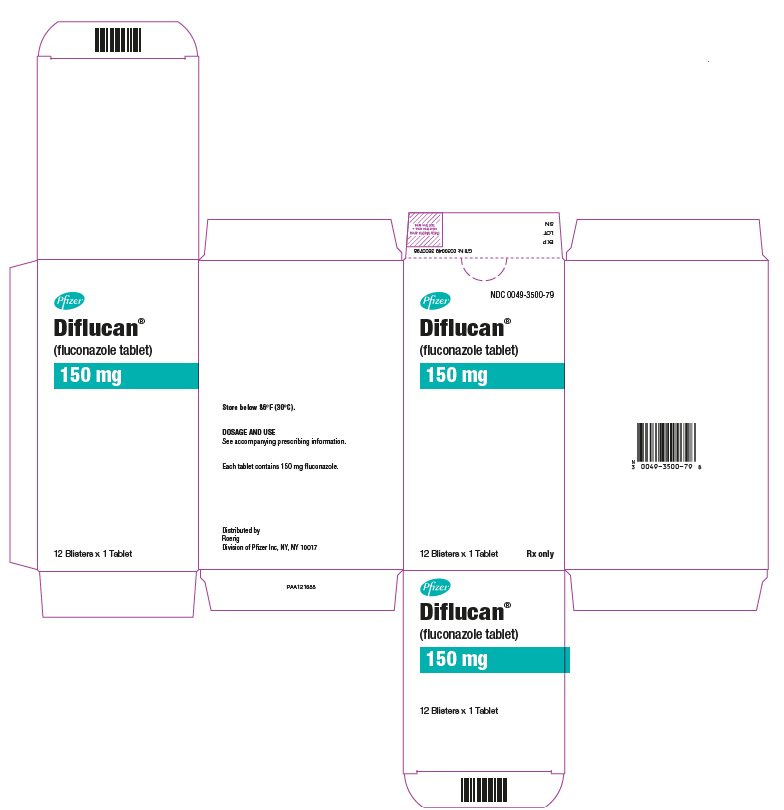
13PRINCIPAL DISPLAY PANEL - 12 x 150 mg Tablet Blister Pack Carton
Pfizer
NDC 0049-3500-79
Diflucan®
(fluconazole tablet)
150 mg
(fluconazole tablet)
150 mg
12 Blisters x 1 Tablet
14PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle Label
Pfizer
NDC 0049-3430-30
DIFLUCAN
(Fluconazole Tablets)
(Fluconazole Tablets)
200 mg
30 Tablets

15PRINCIPAL DISPLAY PANEL - 200 mg Tablet Blister Pack
DIFLUCAN®
(Fluconazole Tablet)
(Fluconazole Tablet)
200 mg
Distributed by
13041700
EXP & LOT AREA

16PRINCIPAL DISPLAY PANEL - 200 mg Tablet Blister Pack Carton
UNIT DOSE
Pfizer
NDC 0049-3430-41
DIFLUCAN®
(Fluconazole Tablets)
(Fluconazole Tablets)
200 mg
For in-institution use only
100 Tablets
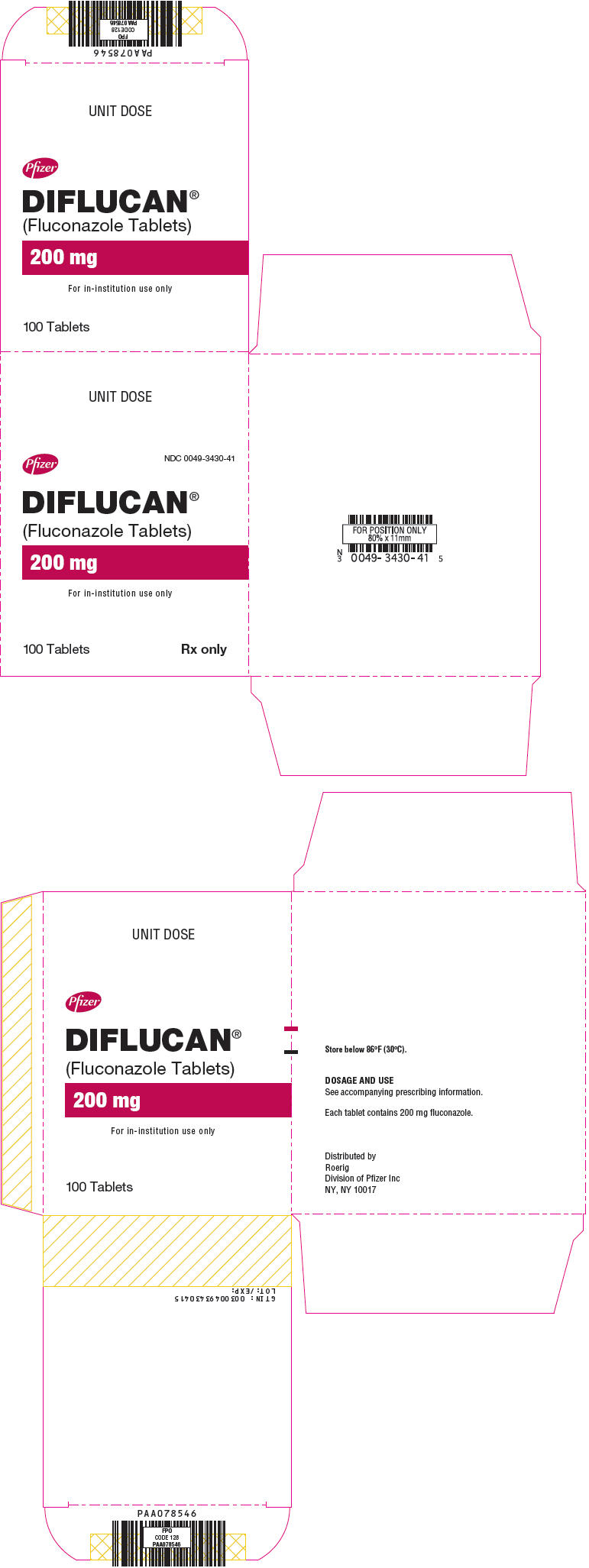
17PRINCIPAL DISPLAY PANEL - 10 mg/mL Bottle Label
Pfizer
NDC 0049-3440-19
DIFLUCAN®
(fluconazole) for Oral
Suspension
(fluconazole) for Oral
Suspension
10 mg/mL
ORANGE FLAVORED
35 mL when reconstituted
35 mL when reconstituted
Rx only

18PRINCIPAL DISPLAY PANEL - 10 mg/mL Bottle Carton
Pfizer
NDC 0049-3440-19
DIFLUCAN®
(fluconazole) for Oral
Suspension
(fluconazole) for Oral
Suspension
10 mg/mL
ORANGE FLAVORED
35 mL when reconstituted
Rx only

19PRINCIPAL DISPLAY PANEL - 40 mg/mL Bottle Label
Pfizer
NDC 0049-3450-19
DIFLUCAN®
(fluconazole) for Oral
Suspension
(fluconazole) for Oral
Suspension
40 mg/mL
ORANGE FLAVORED
35 mL when reconstituted
35 mL when reconstituted
Rx only

20PRINCIPAL DISPLAY PANEL - 40 mg/mL Bottle Carton
Pfizer
NDC 0049-3450-19
DIFLUCAN®
(fluconazole) for Oral
Suspension
(fluconazole) for Oral
Suspension
40 mg/mL
ORANGE FLAVORED
35 mL when reconstituted
Rx only
