Brand Name
Zegalogue
Generic Name
Dasiglucagon
View Brand Information FDA approval date: April 05, 2021
Classification: Antihypoglycemic Agent
Form: Injection
What is Zegalogue (Dasiglucagon)?
ZEGALOGUE ® is indicated for the treatment of severe hypoglycemia in pediatric and adult patients with diabetes aged 6 years and above. ZEGALOGUE is an antihypoglycemic agent indicated for the treatment of severe hypoglycemia in pediatric and adult patients with diabetes aged 6 years and above.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Zegalogue (dasiglucagon)
1INDICATIONS AND USAGE
ZEGALOGUE
2DOSAGE FORMS AND STRENGTHS
ZEGALOGUE injection is a clear, colorless solution available as:
- 0.6 mg/0.6 mL single-dose autoinjector
- 0.6 mg/0.6 mL single-dose prefilled syringe
3CONTRAINDICATIONS
ZEGALOGUE is contraindicated in patients with:
- Pheochromocytoma because of the risk of substantial increase in blood pressure
- Insulinoma because of the risk of hypoglycemia
4ADVERSE REACTIONS
The following important adverse reactions are described elsewhere in the labeling:
- Hypersensitivity and Allergic Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of ZEGALOGUE cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials, 316 adult patients with type 1 diabetes and 20 pediatric patients with type 1 diabetes were exposed to ZEGALOGUE.
The data in Table 1 reflect exposure of 116 adult patients to ZEGALOGUE in 2 placebo-controlled trials (mean age 40 years). Table 2 reflects exposure of 20 pediatric patients exposed to ZEGALOGUE in a placebo-controlled trial. Eight patients were 7 to 11 years old, and 12 were 12 to 17 years old
4.2Immunogenicity
As with all therapeutic peptides, there is a potential for immunogenicity with ZEGALOGUE. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to ZEGALOGUE with the incidence of antibodies to other products may be misleading.
In clinical trials, 4/498 (<1%) of ZEGALOGUE-treated patients developed treatment-emergent anti-drug antibodies (ADAs). Two patients receiving a single dose of ZEGALOGUE had detectable ADAs to dasiglucagon for at least 14 months after dosing. One ADA-positive patient receiving multiple doses of ZEGALOGUE had ADAs with transient neutralizing activity and with cross-reactivity against native glucagon. Although no safety or efficacy concerns were noted for these ADA-positive subjects, it is unknown whether ADAs may affect pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of the drug
5OVERDOSAGE
If overdosage occurs, the patient may experience nausea, vomiting, inhibition of GI tract motility, and/or increases in blood pressure and heart rate. In case of suspected overdosing, serum potassium may decrease and should be monitored and corrected if needed. If the patient develops a marked increase in blood pressure, phentolamine mesylate has been shown to be effective in lowering blood pressure for the short time that control would be needed.
Appropriate supportive treatment should be initiated according to the patient's clinical signs and symptoms.
6DESCRIPTION
ZEGALOGUE contains dasiglucagon hydrochloride, which is a glucagon analog and an antihypoglycemic agent. Dasiglucagon is comprised of 29 amino acids. The molecular formula of dasiglucagon (anhydrous, free-base) is C

ZEGALOGUE injection is a preservative free, sterile, aqueous, clear, and colorless solution for subcutaneous use in a single-dose prefilled syringe and an autoinjector. Each prefilled syringe and autoinjector contains 0.63 mg of dasiglucagon provided as dasiglucagon hydrochloride, which is a salt with 3 - 5 equivalents of hydrochloride, and contains the following inactive ingredients: 3.82 mg tromethamine, 6.44 mg sodium chloride, and water for injection. Hydrochloric acid and/or sodium hydroxide may have been added to adjust pH to 6.5.
7CLINICAL STUDIES
Three randomized, double-blind, placebo-controlled, multicenter trials were conducted in patients with type 1 diabetes. Two trials (Trial A and Trial B) were conducted in adult patients, and one trial (Trial C) was conducted in pediatric patients aged 6 to 17 years. In all 3 trials, patients were randomized to ZEGALOGUE 0.6 mg, placebo, or (in Trials A and C) glucagon for injection 1.0 mg. ZEGALOGUE and the comparators were administered as single subcutaneous injections following a controlled induction of hypoglycemia using intravenous administration of insulin. During this procedure, a plasma glucose concentration of <60 mg/dL was targeted in Trials A and B, whereas the target was <80 mg/dL in Trial C.
The primary efficacy endpoint for all 3 trials was time to plasma glucose recovery (treatment success), defined as an increase in blood glucose of ≥20 mg/dL from time of administration, without additional intervention within 45 minutes. In Trials A and B, plasma glucose values were collected and assessed at pre-dose, and at 4, 6, 8, 10, 12, 15, 17, 20, 25, 30, 40, 45, 50, 60, 75, 90 minutes after treatment. Trial C assessed plasma glucose at the same timepoints as did Trials A and B, with the exception of the 25, 40, 50, 75 and 90-minute post-treatment timepoints. The primary hypothesis test was superiority of ZEGALOGUE versus placebo. There was no formal hypothesis test of ZEGALOGUE versus glucagon for injection.
7.1Adult Patients
Trial A, NCT03378635: A total of 170 patients were randomized 2:1:1 to ZEGALOGUE, placebo, and glucagon for injection, stratified by injection sites (abdominal region, buttocks, thigh). The mean age of the patients was 39.1 years (96% were < 65 years), and the mean duration of diabetes was 20.0 years; 63% were male; 92% were White. The mean baseline plasma glucose was 58.8 mg/dL. The median time to plasma glucose recovery was statistically significantly shorter for ZEGALOGUE (10 minutes) versus placebo (40 minutes) (Table 4). Figure 3 shows the cumulative proportions of patients achieving plasma glucose recovery over time. The median time to plasma glucose recovery was numerically similar between ZEGALOGUE (10 minutes) and glucagon for injection (12 minutes).
Trial B, NCT03688711: A total of 45 patients were randomized 3:1 to ZEGALOGUE and placebo stratified by injection sites (buttocks, deltoid). The mean age of the patients was 41.0 years (95% were < 65 years), and the mean duration of diabetes was 22.5 years; 57% were male; 93% were White. The mean baseline plasma glucose was 55.0 mg/dL. The median time to plasma glucose recovery was statistically significantly shorter for ZEGALOGUE (10 minutes) versus placebo (35 minutes) (Table 4).
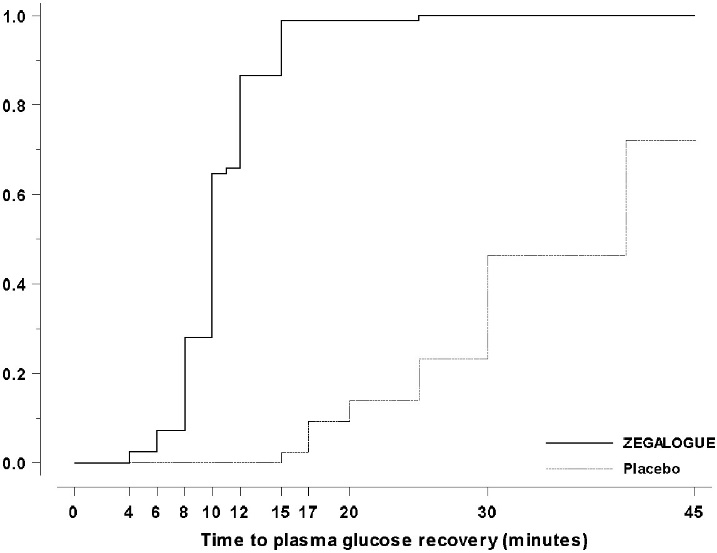
- Figure 3 Time to plasma glucose recovery in Trial A
7.2Pediatric Patients
Trial C, NCT03667053: Pediatric patients aged 6 to 17 years and weighing ≥20 kg, were randomized 2:1:1 to ZEGALOGUE, placebo, and glucagon for injection, stratified by injection sites (abdominal region, thigh) and age groups (6-11 years and 12-17 years). A total of 42 patients were randomized. The mean age was 12.5 years (range 7 to 17 years), and the mean duration of diabetes was 5.9 years; 56% were male; 95% were White. The mean baseline plasma glucose was 72.0 mg/dL. The median time to plasma glucose recovery was statistically significantly shorter for ZEGALOGUE (10 minutes) versus placebo (30 minutes) (Table 5). Figure 4 shows the cumulative proportions of pediatric patients achieving plasma glucose recovery over time. The median time to plasma glucose recovery was numerically similar between ZEGALOGUE (10 minutes) and glucagon for injection (10 minutes).
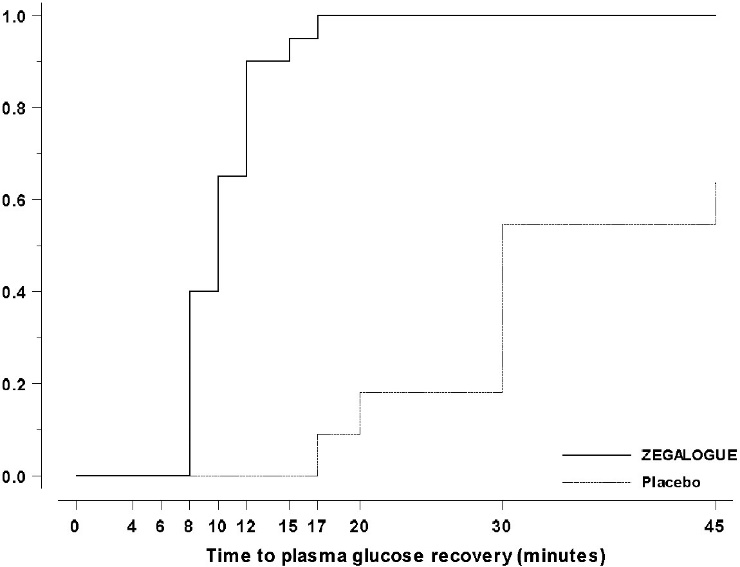
Figure 4. Time to plasma glucose recovery in Trial C
8PATIENT COUNSELING INFORMATION
Advise the patient and family members or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions For Use).
9INSTRUCTIONS FOR USE
ZEGALOGUE
(dasiglucagon) injection
(dasiglucagon) injection
0.6 mg/0.6 mL
Emergency Use for Very Low Blood Sugar
Single-Dose
Prefilled Syringe
Injection, for subcutaneous use
Single-Dose
Prefilled Syringe
Injection, for subcutaneous use
Important: ZEGALOGUE is used to treat very low blood sugar (severe hypoglycemia) where you need help from others.
ZEGALOGUE contains 1 dose of dasiglucagon in a prefilled syringe and cannot be reused.
Read and understand this Instructions For Use before an emergency happens.
Show your family and friends where you keep ZEGALOGUE and explain how to use it by sharing these instructions, so they know how to use ZEGALOGUE before an emergency happens.
The inside of the gray needle cover contains dry natural rubber, which may cause allergic reactions in people with latex allergy.
Hypoglycemia may happen again after receiving ZEGALOGUE treatment.
Early symptoms of hypoglycemia may include:
This Instructions For Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
Novo Nordisk Inc., Plainsboro, NJ, USA
Manufactured by:
Rechon Life Science AB, Malmö, Sweden
Revised 01/2023
10INSTRUCTIONS FOR USE
ZEGALOGUE
(dasiglucagon) injection
0.6 mg/0.6 mL
Emergency Use for Very Low Blood Sugar
Single-Dose
Autoinjector
Injection, for subcutaneous use
Important: ZEGALOGUE is used to treat very low blood sugar (severe hypoglycemia) where you need help from others.
ZEGALOGUE contains 1 dose of dasiglucagon in a prefilled autoinjector and cannot be reused.
Read and understand this Instructions For Use before an emergency happens.
Show your family and friends where you keep ZEGALOGUE and explain how to use it by sharing these instructions, so they know how to use ZEGALOGUE before an emergency happens.
The inside of the gray cap contains dry natural rubber, which may cause allergic reactions in people with latex allergy.
Hypoglycemia may happen again after receiving ZEGALOGUE treatment.
Early symptoms of hypoglycemia may include:
This Instructions For Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
Novo Nordisk Inc., Plainsboro, NJ, USA
Manufactured by:
Rechon Life Science AB, Malmö, Sweden
Revised 01/2023
11PRINCIPAL DISPLAY PANEL - 0.6 mg/0.6 mL Prefilled Syringe 1ct Carton
Emergency Use for Very Low Blood Sugar
ZEGALOGUE
(dasiglucagon) injection
0.6 mg/0.6 mL*
Prefilled Syringe
Single-Dose
Injection, for subcutaneous use. Rx only
NDC 0169-1913-01
List: 191301
Carton contents:
1 Single-Dose Prefilled Syringe
1 Prescribing Information
OPEN HERE
Novo Nordisk

12PRINCIPAL DISPLAY PANEL - 0.6 mg/0.6 mL Prefilled Syringe 2ct Carton
Emergency Use for Very Low Blood Sugar
ZEGALOGUE
(dasiglucagon) injection
0.6 mg/0.6 mL*
Prefilled Syringe
2 Single-Dose Prefilled Syringes
- Injection, for subcutaneous use. Rx only Novo Nordisk
NDC 0169-1913-02
List: 191302
Carton contents:
2 Single-Dose Prefilled Syringes
2 Prescribing Information
- OPEN HERE 2 PACK

13Package/Label Display Panel - 0.6 mg/0.6 mL 1ct Autoinjector Carton
Emergency Use for Very Low Blood Sugar
ZEGALOGUE
(dasiglucagon) injection
0.6 mg/0.6 mL*
Autoinjector
- Single-Dose Injection, for subcutaneous use. Rx only
NDC 0169-1912-01
List: 191201
Carton contents:
1 Single-Dose Autoinjector
1 Prescribing Information
- OPEN HERE Novo Nordisk

14Package/Label Display Panel - 0.6 mg/0.6 mL 2ct Autoinjector Carton
Emergency Use for Very Low Blood Sugar
ZEGALOGUE
(dasiglucagon) injection
0.6 mg/0.6 mL*
Autoinjector
2 Single-Dose Autoinjectors
- Injection, for subcutaneous use. Rx only Novo Nordisk
NDC 0169-1912-02
List: 191202
Carton contents:
2 Single-Dose Autoinjectors
2 Prescribing Information
- OPEN HERE 2 PACK