Choriogonadotropin
What is Ovidrel (Choriogonadotropin)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Immune checkpoint inhibitors (ICI) have revolutionized the management of advanced cancers. However, most rare cancers have been excluded from this progress due to the lack of clinical trials involving these diseases. After the standard first-line treatment, there are no other validated treatments for most of them. The management of these patients in ≥ 2nd line treatment relies on historic poorly e...
Summary: The goal of this clinical trial is to compare the clinical outcomes using different cetrorelix acetate in the context of a GnRH antagonist protocol for ovarian stimulation in women undergoing IVF or intracytoplasmic sperm injection(ICSI) treatment.The main question it aims to answer is whether the generic cetrorelix acetate is non-inferior to the reference product in the GnRH antagonist based prot...
Summary: The aim of this randomized controlled trial is to further examine the possible effects of low dose human chorionic gonadotropin (hCG) priming for eight weeks in women with diminished ovarian reserve (DOR) undergoing in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI). The investigators want to retest the findings of our first study in an identical paired design (NCT04643925), as a...
Related Latest Advances
Brand Information
- Before treatment with gonadotropins is instituted, a thorough gynecologic and endocrinologic evaluation must be performed. This should include an assessment of pelvic anatomy. Patients with tubal obstruction should receive Ovidrel
- Primary ovarian failure should be excluded by the determination of gonadotropin levels.
- Appropriate evaluation should be performed to exclude pregnancy.
- Patients in later reproductive life have a greater predisposition to endometrial carcinoma as well as a higher incidence of anovulatory disorders. A thorough diagnostic evaluation should always be performed in patients who demonstrate abnormal uterine bleeding or other signs of endometrial abnormalities before starting FSH and Ovidrel
- Evaluation of the partner's fertility potential should be included in the initial evaluation.
- Prior hypersensitivity to hCG preparations or one of their excipients.
- Primary ovarian failure.
- Uncontrolled thyroid or adrenal dysfunction.
- An uncontrolled organic intracranial lesion such as a pituitary tumor.
- Abnormal uterine bleeding of undetermined origin (see
- Ovarian cyst or enlargement of undetermined origin (see
- Sex hormone dependent tumors of the reproductive tract and accessory organs.
- Pregnancy.
- Spontaneous Abortion
- Ectopic Pregnancy
- Premature Labor
- Postpartum Fever
- Congenital Abnormalities
- Pulmonary and vascular complications (see
- Adnexal torsion (as a complication of ovarian enlargement)
- Mild to moderate ovarian enlargement
- Hemoperitoneum
- Cases of allergic reactions, including anaphylactic reactions and mild reversible skin rashes have been reported in patients treated with Ovidrel
- Thromboembolic events both in association with, and separate from, the Ovarian Hyperstimulation Syndrome (see "
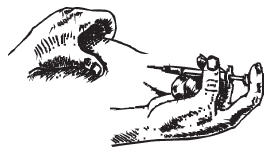
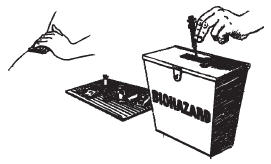
- 1 pre-filled syringe containing 250 μg Ovidrel
