Brand Name
Cuvrior
Generic Name
Trientine Tetrahydrochloride
View Brand Information FDA approval date: September 14, 2022
Classification: Copper Chelator
Form: Tablet
What is Cuvrior (Trientine Tetrahydrochloride)?
CUVRIOR is indicated for the treatment of adult patients with stable Wilson's disease who are de-coppered and tolerant to penicillamine. CUVRIOR is a copper chelator indicated for the treatment of adult patients with stable Wilson's disease who are de-coppered and tolerant to penicillamine.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
CUVRIOR (TRIENTINE TETRAHYDROCHLORIDE)
1INDICATIONS AND USAGE
CUVRIOR is indicated for the treatment of adult patients with stable Wilson's disease who are de-coppered and tolerant to penicillamine.
2DOSAGE FORMS AND STRENGTHS
Tablets: 300 mg of trientine tetrahydrochloride (equivalent to 150 mg of trientine), oblong, yellow coated, functionally scored, printed with OL75 on each side of score line in black ink. Each large carton contains nine small cartons, each containing a blister pack of 8 tablets (a total of 72 tablets in the large carton).
3CONTRAINDICATIONS
CUVRIOR is contraindicated in patients with hypersensitivity to trientine or to any of the excipients in CUVRIOR
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Potential for Worsening of Clinical Symptoms at Initiation of Therapy
- Copper Deficiency
- Iron Deficiency
- Hypersensitivity Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of trientine hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- Gastrointestinal Disorders: Colitis
- Musculoskeletal and Connective Tissue Disorders: Muscle spasms, Rhabdomyolysis
- Nervous System Disorders: Dystonia, Myasthenia gravis
5OVERDOSAGE
Occasional cases of trientine overdose have been reported. A large overdose of 60 g of trientine hydrochloride (equivalent to 80 g CUVRIOR) resulted in nausea, vomiting, dizziness, mild acute kidney injury, mild hypophosphatemia, low serum zinc, and low serum copper. The patient recovered following intravenous hydration and supportive measures.
There is no antidote for trientine acute overdose.
Chronic use of trientine hydrochloride at dosages above the maximum recommended dosage has resulted in sideroblastic anemia.
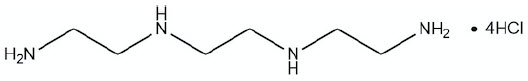
6DESCRIPTION
CUVRIOR contains trientine tetrahydrochloride which is a salt of trientine, a copper chelator. The structural formula of trientine tetrahydrochloride is:
CUVRIOR (trientine tetrahydrochloride) tablets are for oral administration and contain 300 mg of trientine tetrahydrochloride (equivalent to 150 mg trientine). Tablets include the following inactive ingredients: colloidal silicon dioxide, glyceryl dibehenate, and mannitol. The film coating comprises ferric oxide yellow, glyceryl monocaprylocaprate (Type I), polyvinyl alcohol, purified talc, sodium lauryl sulfate, and titanium dioxide.
7CLINICAL STUDIES
The effectiveness of CUVRIOR for the treatment of adult patients with stable Wilson's disease who are decoppered and tolerant to penicillamine was demonstrated in a phase 3 trial (Trial 1). In addition, the safety and effectiveness of CUVRIOR in Wilson's Disease is further supported by studies of another trientine product in patients intolerant to penicillamine.
Trial 1 was a randomized, active-controlled, multi-center, non-inferiority study (NCT03539952) conducted in 53 adult patients with Wilson's disease. The objective was to compare treatment with CUVRIOR to treatment with penicillamine. All patients had been receiving penicillamine for at least 1 year prior to study entry, were adequately controlled and tolerating penicillamine, and had a serum NCC level between ≥ 25 and ≤ 150 mcg/L.
At the start of the study, patients entered a 12-week baseline period and continued to receive their established total daily dosage of penicillamine for 12 weeks. At Week 12, patients were randomized to either remain on penicillamine (N=27) or to switch to CUVRIOR (N=26) for the 24-week post-randomization period (i.e., Week 12 through Week 36). For patients switching to CUVRIOR, where possible, the initial total daily dosage was determined as the trientine base in mg that was the same as the patient's total daily dosage in mg of penicillamine. Where a direct mg to mg conversion was not possible, the total daily dosage of CUVRIOR was rounded to the nearest 150 mg of trientine base (300 mg trientine tetrahydrochloride salt) to the penicillamine total daily dosage. The dosage was permitted to be adjusted depending on clinical response. The mean CUVRIOR total daily dosage was 1,800 mg. Upon switching from penicillamine to CUVRIOR, 3 patients switched to a CUVRIOR total daily dosage < 900 mg, 9 patients to a total daily dosage between 900 mg and 1,800 mg, and 14 patients to a total daily dosage of 1,800 mg or greater. Three out of 26 patients increased and one patient reduced the total daily dosage across the 24-week post-randomization period.
The results are presented in Table 5. The primary efficacy endpoint was the mean serum non-ceruloplasmin copper (NCC) level at 24 weeks post-randomization (Week 36). At Week 12 (prior to initiation of randomized treatment), the mean (95% CI) NCC levels in the penicillamine and CUVRIOR arms were 77 mcg/L (66; 88) and 66 mcg/L (55; 76), respectively. The mean NCC level at Week 36 as measured using an assay not commercially available was similar in patients receiving CUVRIOR and in patients receiving penicillamine. However, the mean 24-hour urinary copper excretion (UCE) at Week 36 was lower in patients receiving CUVRIOR as compared to patients receiving penicillamine. A decrease in UCE has been observed when switching patients from penicillamine products to trientine products. All patients in both treatment arms were considered clinically stable as determined by an adjudication committee at Week 36.
8HOW SUPPLIED/STORAGE AND HANDLING
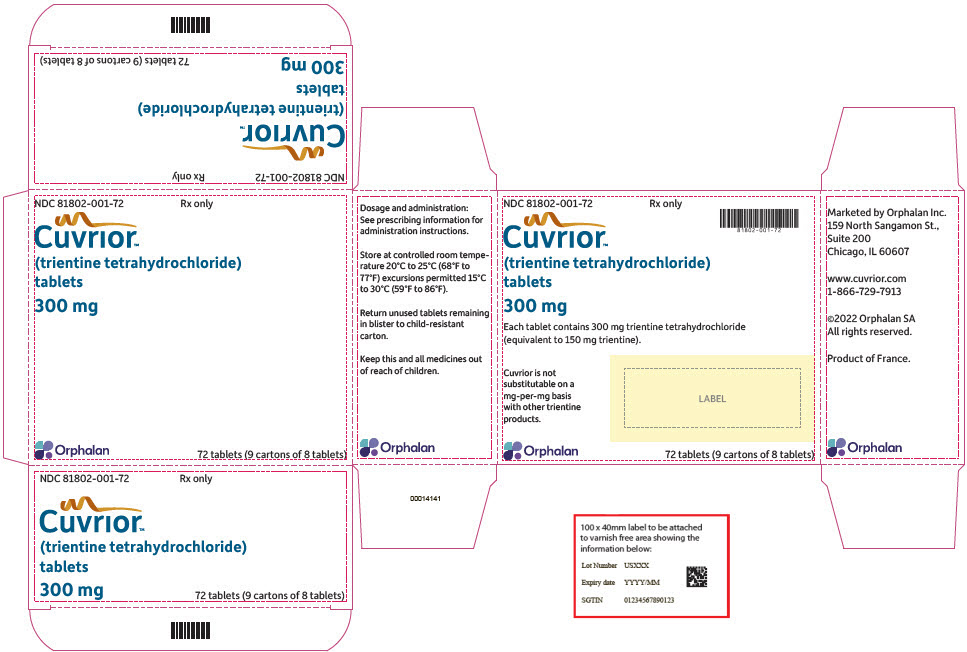
CUVRIOR tablets, 300 mg of trientine tetrahydrochloride, are oblong, yellow coated, functionally scored, and imprinted with OL75 on each side. Each large carton (NDC 81802-001-72) contains nine child-resistant small cartons (NDC 81802-001-08), each containing a blister pack of 8 tablets (a total of 72 tablets in the large carton). The fewest number of tablets that can be dispensed is 8 tablets in a small carton.
Do not remove tablets from the blister pack until the time of dosing.
9PRINCIPAL DISPLAY PANEL - 72 Tablet Blister Pack Carton
NDC 81802-001-72
Cuvrior
(trientine tetrahydrochloride)
300 mg
Orphalan