Cyltezo
What is Cyltezo (Adalimumab-adbm)?
Related Clinical Trials
Summary: The purpose of this study is to estimate and compare the incidence of overall malignancy, serious infection, and opportunistic infections between new users of ustekinumab and new users of other biologic therapies among adult participants with Crohn's disease (CD) or ulcerative colitis (UC).
Summary: HS is relatively common in the United States with a prevalence of 0.1-1.0%. 1 HS has a dramatic impact on quality of life, significantly more so than other chronic skin diseases, such as psoriasis or atopic dermatitis (AD). HS also has a large economic impact, due to frequent emergency department and inpatient care utilization, and re-hospitalization rates similar to congestive heart failure. Unfo...
Summary: The aim of this study is to establish comparability of pharmacokinetic parameters and similarity of the safety and immunogenicity profiles of single subcutaneous doses of BCD 057 100 mg/mL and BCD-057 50 mg/mL, as well as BCD-057 100 mg/mL and Humira 100 mg/mL, in healthy subjects. The study is conducted in a population of healthy male subjects aged 18-45 years inclusive at the time of signing the...
Related Latest Advances
Brand Information
- Serious Infections
- Malignancies
- Hypersensitivity Reactions
- Hepatitis B Virus Reactivation
- Neurologic Reactions
- Hematological Reactions
- Heart Failure
- Autoimmunity
- National Cancer Institute. Surveillance, Epidemiology, and End Results Database (SEER) Program. SEER Incidence Crude Rates, 17 Registries, 2000-2007.
- Do not use a CYLTEZO Pen until you have been shown the right way to give the injections and have read and understood this Instructions for Use. If your doctor decides that you or a caregiver may be able to give your injections of CYLTEZO at home, you should receive training on the right way to prepare and inject CYLTEZO. To help you remember when to inject CYLTEZO, you can mark your calendar ahead of time.
- Do not remove the cap until you are ready to inject.
- Store CYLTEZO in the refrigerator at 36°F to 46°F (2°C to 8°C). Store CYLTEZO in the original carton until use to protect it from light.
- Do not freeze CYLTEZO. Do not use CYLTEZO if frozen, even if it has been thawed.
- Refrigerated CYLTEZO may be used until the expiration date printed on the CYLTEZO carton, dose tray, or pen. Do not use CYLTEZO after the expiration date.
- If needed, for example, when you are traveling, you may also store CYLTEZO at room temperature up to 77°F (25°C) for up to 14 days. Store CYLTEZO in the original carton until use to protect it from light.
- Throw away CYLTEZO if it has been kept at room temperature and not been used within 14 days.
- Record the date you first remove CYLTEZO from the refrigerator in the spaces provided on the carton and dose tray.
- Do not store CYLTEZO in extreme heat or cold.
- Do not use a CYLTEZO Pen if the liquid is milky, discolored, or has flakes or particles in it.
- Do not drop or crush CYLTEZO. The prefilled syringe inside the pen is glass.
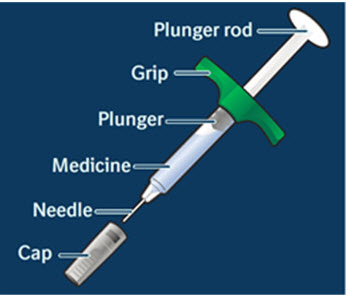
- Do not use a CYLTEZO prefilled syringe until you have been shown the right way to give the injections and have read and understood this Instructions for Use. If your doctor decides that you or a caregiver may be able to give your injections at home, you should receive training on the right way to prepare and give the injection. To help you remember when to inject CYLTEZO, you can mark your calendar ahead of time.
- Do not remove the cap until you are ready to inject.
- Store CYLTEZO in the refrigerator at 36°F to 46°F (2°C to 8°C). Store CYLTEZO in the original carton until use to protect it from light.
- Do not freeze CYLTEZO. Do not use CYLTEZO if frozen, even if it has been thawed.
- Refrigerated CYLTEZO may be used until the expiration date printed on the CYLTEZO carton, dose tray, or prefilled syringe. Do not use CYLTEZO after the expiration date.
- If needed, for example, when you are traveling, you may also store CYLTEZO at room temperature up to 77°F (25°C) for up to 14 days. Store CYLTEZO in the original carton until use to protect it from light.
- Throw away CYLTEZO if it has been kept at room temperature and not been used within 14 days.
- Record the date you first remove CYLTEZO from the refrigerator in the spaces provided on the carton and dose tray.
- Do not store CYLTEZO in extreme heat or cold.
- Do not use a prefilled syringe if the liquid is milky, discolored, or has flakes or particles in it.
- Do not drop or crush CYLTEZO. The prefilled syringe is glass.