Brand Name
Nexviazyme
Generic Name
Avalglucosidase Alfa-Ngpt
View Brand Information FDA approval date: August 06, 2021
Classification: Hydrolytic Lysosomal Glycogen-specific Enzyme
Form: Injection
What is Nexviazyme (Avalglucosidase Alfa-Ngpt)?
NEXVIAZYME is indicated for the treatment of patients 1 year of age and older with late-onset Pompe disease (lysosomal acid alpha-glucosidase deficiency). NEXVIAZYME is a hydrolytic lysosomal glycogen-specific enzyme indicated for the treatment of patients 1 year of age and older with late-onset Pompe disease (lysosomal acid alpha-glucosidase deficiency).
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Descriptive Safety Study Based on Data Collected From Women and Their Offspring Exposed to Nexviazyme/Nexviadyme (Avalglucosidase Alfa-ngpt/Avalglucosidase Alfa) During Pregnancy and/or Lactation in the Postmarketing Setting
Summary: This is a worldwide, descriptive safety study collecting data on women and their offspring exposed to avalglucosidase alfa during pregnancy and/or lactation, to assess the risks of avalglucsodiase alfa on pregnancy and maternal complications and adverse effects in the developing fetus, neonate, and infant. * Outcomes in exposed infants, including growth and development, will be assessed through at...
Related Latest Advances
Brand Information
Nexviazyme (avalglucosidase alfa-ngpt)
1INDICATIONS AND USAGE
NEXVIAZYME is indicated for the treatment of patients 1 year of age and older with late-onset Pompe disease (lysosomal acid alpha-glucosidase [GAA] deficiency).
2DOSAGE FORMS AND STRENGTHS
For injection: 100 mg of avalglucosidase alfa-ngpt as a white to pale-yellow lyophilized powder in a single-dose vial for reconstitution.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Hypersensitivity Reactions Including Anaphylaxis
- Infusion-Associated Reactions (IARs)
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
5DESCRIPTION
Avalglucosidase alfa-ngpt is a hydrolytic lysosomal glycogen-specific recombinant human α-glucosidase enzyme conjugated with multiple synthetic bis-mannose-6-phosphate (bis-M6P)-tetra-mannose glycans resulting in approximately 15 moles of M6P per mole of enzyme (15 M6P) and is produced in Chinese hamster ovary cells (CHO). Avalglucosidase alfa-ngpt has a molecular weight of approximately 124 kDa.
NEXVIAZYME (avalglucosidase alfa-ngpt) for injection is a sterile white to pale-yellow lyophilized powder for intravenous use after reconstitution and dilution. Each single-dose vial contains 100 mg of avalglucosidase alfa-ngpt, glycine (200 mg), L-Histidine (10.7 mg), L-Histidine HCl monohydrate (6.5 mg), mannitol (200 mg), and polysorbate 80 (1 mg). After reconstitution with 10 mL of Sterile Water for Injection, USP, the resultant concentration is 100 mg/10 mL (10 mg/mL) with a pH of approximately 6.2.
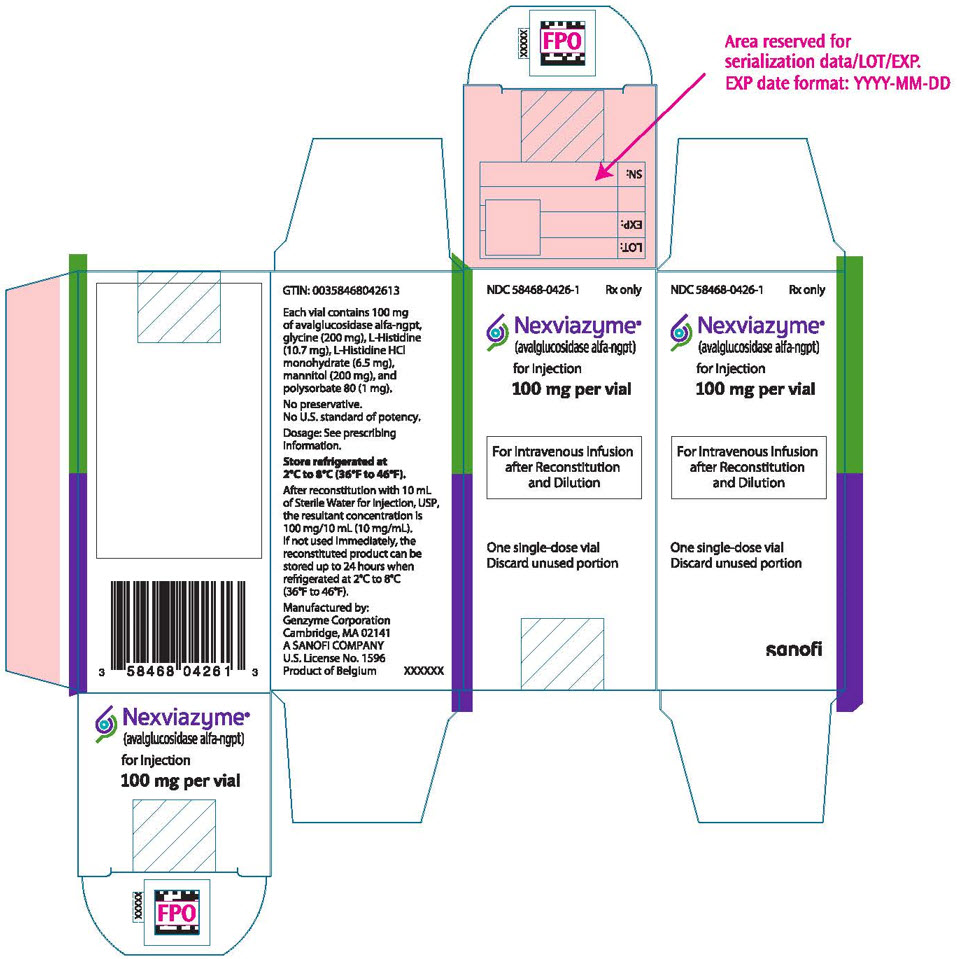
6PRINCIPAL DISPLAY PANEL - 100 mg Vial Carton
NDC 58468-0426-1
Nexviazyme
100 mg per vial
For Intravenous Infusion
One single-dose vial