Brand Name
Lorbrena
Generic Name
Lorlatinib
View Brand Information FDA approval date: November 19, 2018
Classification: Kinase Inhibitor
Form: Tablet
What is Lorbrena (Lorlatinib)?
LORBRENA ® is indicated for the treatment of adult patients with metastatic non-small cell lung cancer whose tumors are anaplastic lymphoma kinase -positive as detected by an FDA-approved test. LORBRENA is a kinase inhibitor indicated for the treatment of adult patients with metastatic non-small cell lung cancer whose tumors are anaplastic lymphoma kinase -positive as detected by an FDA-approved test. ( 1.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Lorbrena (lorlatinib)
1INDICATIONS AND USAGE
LORBRENA
2DOSAGE FORMS AND STRENGTHS
Tablets:
- 25 mg: 8 mm round, tan, immediate release, film-coated, debossed with "Pfizer" on one side and "25" and "LLN" on the other side
- 100 mg: 8.5 mm × 17 mm oval, lavender, immediate release, film-coated, debossed with "Pfizer" on one side and "LLN 100" on the other side
3CONTRAINDICATIONS
LORBRENA is contraindicated in patients taking strong CYP3A inducers, due to the potential for serious hepatotoxicity
4ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling:
- Risk of Serious Hepatotoxicity with Concomitant Use of Strong CYP3A Inducers
- Central Nervous System Effects
- Hyperlipidemia
- Atrioventricular Block
- Interstitial Lung Disease/Pneumonitis
- Hypertension
- Hyperglycemia
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in the Warnings and Precautions section reflects exposure to LORBRENA in 476 patients who received 100 mg LORBRENA once daily in Study B7461001 (N=327) and Study B7461006 (N=149). Among 476 patients who received LORBRENA, 75% were exposed for 6 months or longer and 61% were exposed for greater than 1 year. In this pooled safety population, the most frequent adverse reactions in ≥ 20% of 476 patients who received LORBRENA were edema (56%), peripheral neuropathy (44%), weight gain (31%), cognitive effects (28%), fatigue (27%), dyspnea (27%), arthralgia (24%), diarrhea (23%), mood effects (21%), and cough (21%). The most frequent Grade 3–4 laboratory abnormalities in ≥ 20% of 476 patients who received LORBRENA were hypercholesterolemia (21%) and hypertriglyceridemia (21%).
5DESCRIPTION
LORBRENA (lorlatinib) is a kinase inhibitor for oral administration. The molecular formula is C
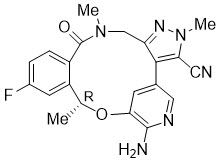
Lorlatinib is a white to off-white powder with a pKa of 4.92. The solubility of lorlatinib in aqueous media decreases over the range pH 2.55 to pH 8.02 from 32.38 mg/mL to 0.17 mg/mL. The log of the distribution coefficient (octanol/water) at pH 9 is 2.45.
LORBRENA is supplied as tablets containing 25 mg or 100 mg of lorlatinib with the following inactive ingredients: microcrystalline cellulose, dibasic calcium phosphate anhydrous, sodium starch glycolate, and magnesium stearate. The film-coating contains hydroxypropyl methylcellulose (HPMC) 2910/hypromellose, lactose monohydrate, macrogol/polyethylene glycol (PEG) 3350, triacetin, titanium dioxide, ferrosoferric oxide/black iron oxide, and iron oxide red.
6HOW SUPPLIED/STORAGE AND HANDLING
Table 11 describes the available strengths and package configurations for LORBRENA:
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
8PRINCIPAL DISPLAY PANEL - 25 mg Tablet Bottle Label Sample
PROFESSIONAL SAMPLE – NOT FOR SALE
Pfizer
Lorbrena
30 Tablets