Generic Name
Celecoxib
Brand Names
Celebrex, Elyxyb, Vyscoxa
FDA approval date: October 02, 1998
Classification: Nonsteroidal Anti-inflammatory Drug
Form: Suspension, Capsule, Liquid
What is Celebrex (Celecoxib)?
Celecoxib Capsule is a non-steroidal anti-inflammatory drug indicated for: Osteoarthritis .
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
CELEBREX (Celecoxib)
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
Cardiovascular Thrombotic Events
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction, and stroke, which can be fatal. This risk may occur early in the treatment and may increase with duration of use
- CELEBREX is contraindicated in the setting of coronary artery bypass graft (CABG) surgery
Gastrointestinal Bleeding, Ulceration, and Perforation
- NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events
1INDICATIONS AND USAGE
CELEBREX is indicated
1.1Osteoarthritis (OA)
For the management of the signs and symptoms of OA
1.2Rheumatoid Arthritis (RA)
For the management of the signs and symptoms of RA
1.3Juvenile Rheumatoid Arthritis (JRA)
For the management of the signs and symptoms of JRA in patients 2 years and older
1.4Ankylosing Spondylitis (AS)
For the management of the signs and symptoms of AS
1.5Acute Pain
For the management of acute pain in adults
1.6Primary Dysmenorrhea
For the management of primary dysmenorrhea
2DOSAGE FORMS AND STRENGTHS
CELEBREX (celecoxib) capsules:
- 50 mg white, with reverse printed white on red band of body and cap with markings of 7767 on the cap and 50 on the body.
- 100 mg white, with reverse printed white on blue band of body and cap with markings of 7767 on the cap and 100 on the body.
- 200 mg white, with reverse printed white on gold band with markings of 7767 on the cap and 200 on the body.
- 400 mg white, with reverse printed white on green band with markings of 7767 on the cap and 400 on the body.
3CONTRAINDICATIONS
CELEBREX is contraindicated in the following patients:
- Known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to celecoxib, any components of the drug product
- History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs, have been reported in such patients
- In the setting of CABG surgery
- In patients who have demonstrated allergic-type reactions to sulfonamides
4ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Cardiovascular Thrombotic Events
- GI Bleeding, Ulceration and Perforation
- Hepatotoxicity
- Hypertension
- Heart Failure and Edema
- Renal Toxicity and Hyperkalemia
- Anaphylactic Reactions
- Serious Skin Reactions
- Hematologic Toxicity
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Of the CELEBREX-treated patients in the pre-marketing controlled clinical trials, approximately 4,250 were patients with OA, approximately 2,100 were patients with RA, and approximately 1,050 were patients with post-surgical pain. More than 8,500 patients received a total daily dose of CELEBREX of 200 mg (100 mg twice daily or 200 mg once daily) or more, including more than 400 treated at 800 mg (400 mg twice daily). Approximately 3,900 patients received CELEBREX at these doses for 6 months or more; approximately 2,300 of these have received it for 1 year or more and 124 of these have received it for 2 years or more.
4.1.1Pre-marketing Controlled Arthritis Trials
Table 1 lists all adverse events, regardless of causality, occurring in ≥2% of patients receiving CELEBREX from 12 controlled studies conducted in patients with OA or RA that included a placebo and/or a positive control group. Since these 12 trials were of different durations, and patients in the trials may not have been exposed for the same duration of time, these percentages do not capture cumulative rates of occurrence.
In placebo- or active-controlled clinical trials, the discontinuation rate due to adverse events was 7.1% for patients receiving CELEBREX and 6.1% for patients receiving placebo. Among the most common reasons for discontinuation due to adverse events in the CELEBREX treatment groups were dyspepsia and abdominal pain (cited as reasons for discontinuation in 0.8% and 0.7% of CELEBREX patients, respectively). Among patients receiving placebo, 0.6% discontinued due to dyspepsia and 0.6% withdrew due to abdominal pain.
4.1.2The following adverse reactions occurred in 0.1% to 1.9% of patients treated with CELEBREX (100 mg to 200 mg twice daily or 200 mg once daily):
Gastrointestinal: Constipation, diverticulitis, dysphagia, eructation, esophagitis, gastritis, gastroenteritis, gastroesophageal reflux, hemorrhoids, hiatal hernia, melena, dry mouth, stomatitis, tenesmus, vomiting
Cardiovascular: Aggravated hypertension, angina pectoris, coronary artery disorder, myocardial infarction
General: Hypersensitivity, allergic reaction, chest pain, cyst NOS, edema generalized, face edema, fatigue, fever, hot flushes, influenza-like symptoms, pain, peripheral pain
Central, peripheral nervous system: Leg cramps, hypertonia, hypoesthesia, migraine, paresthesia, vertigo
Hearing and vestibular: Deafness, tinnitus
Heart rate and rhythm: Palpitation, tachycardia
Liver and biliary: Hepatic enzyme increased (including SGOT increased, SGPT increased)
Metabolic and nutritional: blood urea nitrogen (BUN) increased, creatine phosphokinase (CPK) increased, hypercholesterolemia, hyperglycemia, hypokalemia, NPN increased, creatinine increased, alkaline phosphatase increased, weight increased
Musculoskeletal: Arthralgia, arthrosis, myalgia, synovitis, tendinitis
Platelets (bleeding or clotting): Ecchymosis, epistaxis, thrombocythemia
Psychiatric: Anorexia, anxiety, appetite increased, depression, nervousness, somnolence
Hemic: Anemia
Respiratory: Bronchitis, bronchospasm, bronchospasm aggravated, cough, dyspnea, laryngitis, pneumonia
Skin and appendages: Alopecia, dermatitis, photosensitivity reaction, pruritus, rash erythematous, rash maculopapular, skin disorder, skin dry, sweating increased, urticaria
Application site disorders: Cellulitis, dermatitis contact
Urinary: Albuminuria, cystitis, dysuria, hematuria, micturition frequency, renal calculus
4.1.3The following serious adverse events (causality not evaluated) occurred in <0.1% of patients:
Cardiovascular: Syncope, congestive heart failure, ventricular fibrillation, pulmonary embolism, cerebrovascular accident, peripheral gangrene, thrombophlebitis
Gastrointestinal: Intestinal obstruction, intestinal perforation, gastrointestinal bleeding, colitis with bleeding, esophageal perforation, pancreatitis, ileus
General: Sepsis, sudden death
Liver and biliary: Cholelithiasis
Hemic and lymphatic: Thrombocytopenia
Nervous: Ataxia, suicide [see
Renal: Acute renal failure
4.1.4The Celecoxib Long-Term Arthritis Safety Study [see Clinical Studies (14.7)]
Hematological Events: The incidence of clinically significant decreases in hemoglobin (>2 g/dL) was lower in patients on CELEBREX 400 mg twice daily (0.5%) compared to patients on either diclofenac 75 mg twice daily (1.3%) or ibuprofen 800 mg three times daily 1.9%. The lower incidence of events with CELEBREX was maintained with or without aspirin use [see .
Withdrawals/Serious Adverse Events: Kaplan-Meier cumulative rates at 9 months for withdrawals due to adverse events for CELEBREX, diclofenac and ibuprofen were 24%, 29%, and 26%, respectively. Rates for serious adverse events (i.e., causing hospitalization or felt to be life-threatening or otherwise medically significant), regardless of causality, were not different across treatment groups (8%, 7%, and 8%, respectively).
4.1.5Juvenile Rheumatoid Arthritis Study
In a 12-week, double-blind, active-controlled study, 242 JRA patients 2 years to 17 years of age were treated with celecoxib or naproxen; 77 JRA patients were treated with celecoxib 3 mg/kg twice daily, 82 patients were treated with celecoxib 6 mg/kg twice daily, and 83 patients were treated with naproxen 7.5 mg/kg twice daily. The most commonly occurring (≥5%) adverse events in celecoxib treated patients were headache, fever (pyrexia), upper abdominal pain, cough, nasopharyngitis, abdominal pain, nausea, arthralgia, diarrhea, and vomiting. The most commonly occurring (≥5%) adverse experiences for naproxen-treated patients were headache, nausea, vomiting, fever, upper abdominal pain, diarrhea, cough, abdominal pain, and dizziness (Table 2). Compared with naproxen, celecoxib at doses of 3 and 6 mg/kg twice daily had no observable deleterious effect on growth and development during the course of the 12-week double-blind study. There was no substantial difference in the number of clinical exacerbations of uveitis or systemic features of JRA among treatment groups.
In a 12-week, open-label extension of the double-blind study described above, 202 JRA patients were treated with celecoxib 6 mg/kg twice daily. The incidence of adverse events was similar to that observed during the double-blind study; no unexpected adverse events of clinical importance emerged.
4.2Postmarketing Experience
The following adverse reactions have been identified during post approval use of CELEBREX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure
Cardiovascular: Vasculitis, deep venous thrombosis
General: Anaphylactoid reaction, angioedema
Liver and biliary: Liver necrosis, hepatitis, jaundice, hepatic failure
Hemic and lymphatic: Agranulocytosis, aplastic anemia, pancytopenia, leucopenia
Metabolic: Hypoglycemia, hyponatremia
Nervous: Aseptic meningitis, ageusia, anosmia, fatal intracranial hemorrhage
Renal: Interstitial nephritis
Skin and Appendages: Erythema multiforme, exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP), and fixed drug eruption (FDE)
5DRUG INTERACTIONS
See Table 3 for clinically significant drug interactions with celecoxib.
6OVERDOSAGE
Symptoms following acute NSAID overdosages have been typically limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which have been generally reversible with supportive care. Gastrointestinal bleeding has occurred. Hypertension, acute renal failure, respiratory depression, and coma have occurred, but were rare
No overdoses of CELEBREX were reported during clinical trials. Doses up to 2400 mg/day for up to 10 days in 12 patients did not result in serious toxicity. No information is available regarding the removal of celecoxib by hemodialysis, but based on its high degree of plasma protein binding (>97%) dialysis is unlikely to be useful in overdose.
Manage patients with symptomatic and supportive care following an NSAID overdosage. There are no specific antidotes. Consider emesis and/or activated charcoal (60 to 100 grams in adults, 1 to 2 grams per kg of body weight in pediatric patients) and/or osmotic cathartic in symptomatic patients seen within four hours of ingestion or in patients with a large overdosage (5 to 10 times the recommended dosage). Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding.
For additional information about overdosage treatment contact a poison control center (1-800-222-1222).
7DESCRIPTION
CELEBREX (celecoxib) capsule is a nonsteroidal anti-inflammatory drug, available as capsules containing 50 mg, 100 mg, 200 mg and 400 mg celecoxib for oral administration. The chemical name is 4-[5-(4-methylphenyl)- 3-(trifluoromethyl)-1H-pyrazol-1-yl] benzenesulfonamide and is a diaryl-substituted pyrazole. The molecular weight is 381.38. Its molecular formula is C
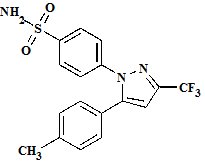
Celecoxib is a white to off-white powder with a pKa of 11.1 (sulfonamide moiety). Celecoxib is hydrophobic (log P is 3.5) and is practically insoluble in aqueous media at physiological pH range.
The inactive ingredients in CELEBREX include: croscarmellose sodium, edible inks, gelatin, lactose monohydrate, magnesium stearate, povidone and sodium lauryl sulfate.
8HOW SUPPLIED/STORAGE AND HANDLING
CELEBREX (celecoxib) 50 mg capsules are white, with reverse printed white on red band of body and cap with markings of 7767 on the cap and 50 on the body, supplied as:
NDC NumberSize58151-082-91 bottle of 60
CELEBREX (celecoxib) 100 mg capsules are white, with reverse printed white on blue band of body and cap with markings of 7767 on the cap and 100 on the body, supplied as:
NDC NumberSize58151-083-01 bottle of 100
58151-083-05 bottle of 500
58151-083-88 carton of 100 unit dose
58151-083-05 bottle of 500
58151-083-88 carton of 100 unit dose
CELEBREX (celecoxib) 200 mg capsules are white, with reverse printed white on gold band with markings of 7767 on the cap and 200 on the body, supplied as:
NDC NumberSize
58151-084-01 bottle of 100
58151-084-05 bottle of 500
58151-084-88 carton of 100 unit dose
58151-084-01 bottle of 100
58151-084-05 bottle of 500
58151-084-88 carton of 100 unit dose
CELEBREX (celecoxib) 400 mg capsules are white, with reverse printed white on green band with markings of 7767 on the cap and 400 on the body, supplied as:
NDC NumberSize
58151-085-91 bottle of 60
58151-085-88 carton of 100 unit dose
58151-085-91 bottle of 60
58151-085-88 carton of 100 unit dose
Storage
Store at room temperature 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide) that accompanies each prescription dispensed. Inform patients, families, or their caregivers of the following information before initiating therapy with CELEBREX and periodically during the course of ongoing therapy.
Cardiovascular Thrombotic Events
Advise patients to be alert for the symptoms of cardiovascular thrombotic events, including chest pain, shortness of breath, weakness, or slurring of speech, and to report any of these symptoms to their health care provider immediately
Gastrointestinal Bleeding, Ulceration, and Perforation
Advise patients to report symptoms of ulcerations and bleeding, including epigastric pain, dyspepsia, melena, and hematemesis to their health care provider. In the setting of concomitant use of low-dose aspirin for cardiac prophylaxis, inform patients of the increased risk for and the signs and symptoms of GI bleeding
Hepatotoxicity
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, diarrhea, jaundice, right upper quadrant tenderness, and “flu-like” symptoms). If these occur, instruct patients to stop CELEBREX and seek immediate medical therapy
Heart Failure and Edema
Advise patients to be alert for the symptoms of congestive heart failure including shortness of breath, unexplained weight gain, or edema and to contact their healthcare provider if such symptoms occur
Anaphylactic Reactions
Inform patients of the signs of an anaphylactic reaction (e.g., difficulty breathing, swelling of the face or throat). Instruct patients to seek immediate emergency help if these occur
Serious Skin Reactions, Including DRESS
Advise patients to stop taking CELEBREX immediately if they develop any type of rash or fever and to contact their healthcare provider as soon as possible
Female Fertility
Advise females of reproductive potential who desire pregnancy that NSAIDs, including CELEBREX, may be associated with a reversible delay in ovulation
Fetal Toxicity
Inform pregnant women to avoid use of CELEBREX and other NSAIDs starting at 30 weeks of gestation because of the risk of the premature closing of the fetal ductus arteriosus. If treatment with CELEBREX is needed for a pregnant woman between about 20 to 30 weeks gestation, advise her that she may need to be monitored for oligohydramnios, if treatment continues for longer than 48 hours
Avoid Concomitant Use of NSAIDs
Inform patients that the concomitant use of CELEBREX with other NSAIDs or salicylates (e.g., diflunisal, salsalate) is not recommended due to the increased risk of gastrointestinal toxicity, and little or no increase in efficacy
Use of NSAIDS and Low-Dose Aspirin
Inform patients not to use low-dose aspirin concomitantly with CELEBREX until they talk to their healthcare provider
Distributed by:
© 2024 Viatris Inc.
CELEBREX is a registered trademark of G.D. Searle LLC, a Viatris Company.
UPJ:CLBRXC:R2mmt
Revised: 11/2024
10Medication Guide
Medication Guide for Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 11/2024
UPJ:MG:CLBRXC:R2m
11PRINCIPAL DISPLAY PANEL – 50 mg
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 58151-082-91
Celebrex
50 mg
60 Capsules
Rx Only
Store at room temperature 20°C
[see USP Controlled
Room Temperature].
[see USP Controlled
Room Temperature].
Dispense in tight
DOSAGE AND USE
See accompanying
prescribing information.
See accompanying
prescribing information.
Each capsule contains
Distributed by:
© 2025 Viatris Inc.
RUPJ082D1

12PRINCIPAL DISPLAY PANEL – 100 mg
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 58151-083-01
Celebrex
100 mg
100 Capsules
Rx Only
Store at room temperature 20°C to 25°C
[see USP Controlled Room Temperature].
[see USP Controlled Room Temperature].
Dispense in tight (USP), child resistant
DOSAGE AND USE
See accompanying prescribing
information.
See accompanying prescribing
information.
Each capsule contains 100 mg celecoxib.
Distributed by:
© 2025 Viatris Inc.
RUPJ083A1

13PRINCIPAL DISPLAY PANEL – 200 mg
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 58151-084-01
Celebrex
200 mg
100 Capsules
Rx Only
Store at room temperature 20°C to 25°C
[see USP Controlled Room Temperature].
[see USP Controlled Room Temperature].
Dispense in tight (USP), child resistant
DOSAGE AND USE
See accompanying prescribing
information.
See accompanying prescribing
information.
Each capsule contains 200 mg celecoxib.
Distributed by:
© 2025 Viatris Inc.
RUPJ084A1

14PRINCIPAL DISPLAY PANEL – 400 mg
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 58151-085-91
Celebrex
400 mg
60 Capsules
Rx Only
Store at room temperature 20°C to 25°C
[see USP Controlled Room Temperature].
[see USP Controlled Room Temperature].
Dispense in tight (USP), child resistant
DOSAGE AND USE
See accompanying prescribing
information.
See accompanying prescribing
information.
Each capsule contains 400 mg celecoxib.
Distributed by:
© 2025 Viatris Inc.
RUPJ085D1
