Brand Name
Brenzavvy
Generic Name
Bexagliflozin
View Brand Information FDA approval date: January 20, 2023
Classification: Sodium-Glucose Cotransporter 2 Inhibitor
Form: Tablet
What is Brenzavvy (Bexagliflozin)?
BRENZAVVY is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Limitations of Use BRENZAVVY is not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus. [see Warnings and Precautions.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Adipose Dysfunction, Imaging, Physiology, and Outcomes With SGLT2i's for Sleep Apnea: The ADIPOSA Study
Summary: The goal of this clinical trial is to test if bexagliflozin lowers the sleep apnea severity in adults who are overweight or obese with moderate to severe obstructive sleep apnea (OSA) compared with a placebo (look-alike substance that contains no active drug). The main question it aims to answer is: * If SGLT2i will reduce anatomic and physiologic traits, clinical measures of OSA and sleep deficie...
Related Latest Advances
Brand Information
BRENZAVVY (Bexagliflozin)
1INDICATIONS AND USAGE
BRENZAVVY is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Limitations of Use
BRENZAVVY is not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus. [see Warnings and Precautions (.
BRENZAVVY is not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus. [see Warnings and Precautions (.
2DOSAGE FORMS AND STRENGTHS
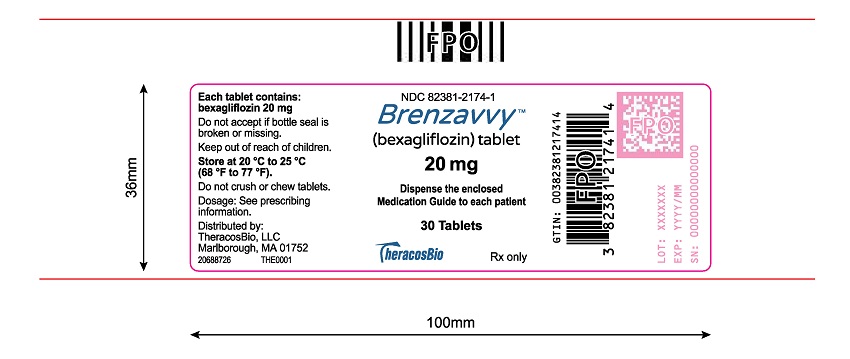
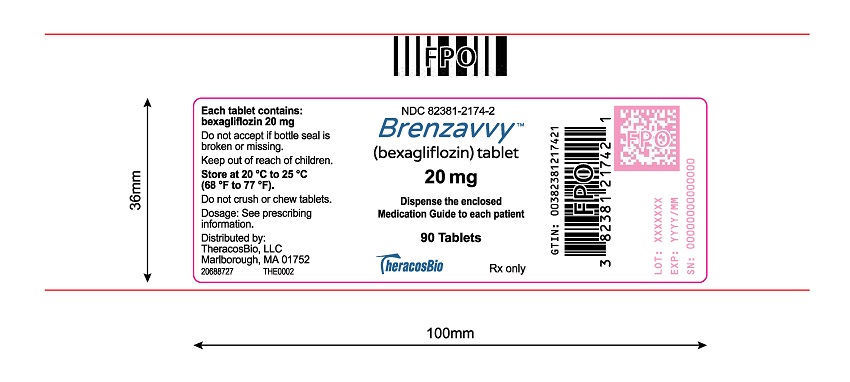
Tablets: 20 mg, blue, caplet-shaped, biconvex, bevel-edged, debossed with “2” and inverted “2” on one side.
3CONTRAINDICATIONS
BRENZAVVY is contraindicated in patients:
- With hypersensitivity to bexagliflozin or any excipient in BRENZAVVY. Anaphylaxis and angioedema have been reported with sodium-glucose co-transporter 2 (SGLT2) inhibitors.
4ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
- Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis
- Lower Limb Amputation
- Volume Depletion
- Genitourinary Infections, including Urosepsis, Pyelonephritis, Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene), and Genital Mycotic Infections
- Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pool of Placebo-Controlled Trials Evaluating BRENZAVVY 20 mg
The data in Table 1 are derived from three trials in adults with type 2 diabetes mellitus: two 24-week placebo-controlled trials (one as monotherapy and another as add-on to metformin therapy; Trials 1 and 2, respectively) [see Clinical Studies ( and a 12-week, placebo-controlled, dose-ranging, monotherapy trial (only the data from the 20 mg dosage of BRENZAVVY per day were included in this pool). In these pooled trials, patients received placebo (N = 300) or BRENZAVVY 20 mg (N = 372), once daily. The mean age of the population was 56 years and 5% of the patients were older than 75 years of age. Fifty-seven percent (57%) were male and 45% were White, 38% Asian, 15% Black and 2% other races. At baseline, the mean duration of type 2 diabetes mellitus was 7.7 years and the mean hemoglobin A1c (HbA1c) was 8.2%. Established microvascular complications of type 2 diabetes mellitus at baseline included diabetic nephropathy (0.8%), retinopathy (24%), and peripheral neuropathy (33%). Baseline renal function was eGFR ≥ 60 mL/min/1.73 m2 in 98% of patients and eGFR 45 to < 60 mL/min/1.73 m2 in 2% of patients (mean eGFR 92 mL/min/1.73 m2).
The data in Table 1 are derived from three trials in adults with type 2 diabetes mellitus: two 24-week placebo-controlled trials (one as monotherapy and another as add-on to metformin therapy; Trials 1 and 2, respectively) [see Clinical Studies ( and a 12-week, placebo-controlled, dose-ranging, monotherapy trial (only the data from the 20 mg dosage of BRENZAVVY per day were included in this pool). In these pooled trials, patients received placebo (N = 300) or BRENZAVVY 20 mg (N = 372), once daily. The mean age of the population was 56 years and 5% of the patients were older than 75 years of age. Fifty-seven percent (57%) were male and 45% were White, 38% Asian, 15% Black and 2% other races. At baseline, the mean duration of type 2 diabetes mellitus was 7.7 years and the mean hemoglobin A1c (HbA1c) was 8.2%. Established microvascular complications of type 2 diabetes mellitus at baseline included diabetic nephropathy (0.8%), retinopathy (24%), and peripheral neuropathy (33%). Baseline renal function was eGFR ≥ 60 mL/min/1.73 m2 in 98% of patients and eGFR 45 to < 60 mL/min/1.73 m2 in 2% of patients (mean eGFR 92 mL/min/1.73 m2).
Table 1 shows common adverse reactions associated with the use of BRENZAVVY in these trials. These adverse reactions occurred more commonly in BRENZAVVY-treated patients than placebo-treated patients, and occurred in at least 2% of BRENZAVVY-treated patients.
Table 1. Adverse Reactions in Adults with Type 2 Diabetes Mellitus - Monotherapy or in Combination with Metformin*
* The three placebo-controlled trials included two monotherapy trials and one add-on combination trial with metformin in adults with type 2 diabetes mellitus (Trials 1, 2, and a 12-week dose ranging trial). Adverse reactions were those that occurred more commonly in BRENZAVVY-treated patients than placebo-treated patients and occurred in at least 2% of BRENZAVVY-treated patients.
a Includes: polyuria, pollakiuria, micturition urgency, nocturia.
b Includes: dysuria, urinary tract infection, nitrite urine present, streptococcal urinary tract infection, cystitis.
c Includes: vulvovaginal mycotic infection, vaginal infection, genital infection fungal, vulvovaginal candidiasis. Percentages calculated with the number of female patients in each group as denominator: placebo (N = 130), BRENZAVVY (N = 156).
d Includes: thirst, polydipsia.
e Includes: pruritus genital, vulvovaginal pruritus. Percentages calculated with the number of females in each group as denominator: placebo (N = 130), BRENZAVVY (N = 156).
f Includes: balanoposthitis, genital infection fungal, tinea cruris. Percentages calculated with the number of males in each group as denominator: placebo (N = 170), BRENZAVVY (N = 216).
b Includes: dysuria, urinary tract infection, nitrite urine present, streptococcal urinary tract infection, cystitis.
c Includes: vulvovaginal mycotic infection, vaginal infection, genital infection fungal, vulvovaginal candidiasis. Percentages calculated with the number of female patients in each group as denominator: placebo (N = 130), BRENZAVVY (N = 156).
d Includes: thirst, polydipsia.
e Includes: pruritus genital, vulvovaginal pruritus. Percentages calculated with the number of females in each group as denominator: placebo (N = 130), BRENZAVVY (N = 156).
f Includes: balanoposthitis, genital infection fungal, tinea cruris. Percentages calculated with the number of males in each group as denominator: placebo (N = 170), BRENZAVVY (N = 216).
Clinical Trial in Patients with Increased Risk for Major Adverse Cardiovascular EventsBRENZAVVY was evaluated in a trial that enrolled adults with type 2 diabetes mellitus who had either established (CVD) or were at increased risk for CVD (Trial 6) [see Clinical Studies (. Patients on standard of care therapy for diabetes management were randomized to receive add-on therapy with either placebo (N = 567) or BRENZAVVY 20 mg once daily (N = 1,132) for a minimum duration of 52 weeks (median duration 2.4 years). The most common adverse reactions observed in this trial were generally consistent with other trials of BRENZAVVY in adults with type 2 diabetes mellitus (see .
Other Adverse Reactions
Lower Limb Amputations
An increased incidence of non-traumatic lower limb amputations occurred in BRENZAVVY-treated patients compared to placebo-treated patients in a trial (Trial 6) that evaluated adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD. Patients in this trial were followed for a median duration of 2.4 years. The lower limb amputation data are shown in Table 2.
Lower Limb Amputations
An increased incidence of non-traumatic lower limb amputations occurred in BRENZAVVY-treated patients compared to placebo-treated patients in a trial (Trial 6) that evaluated adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD. Patients in this trial were followed for a median duration of 2.4 years. The lower limb amputation data are shown in Table 2.
Table 2. Non-traumatic Lower Limb Amputation in Adults with Type 2 Diabetes Mellitus who had either Established Cardiovascular Disease or were at Risk for Cardiovascular Disease (Trial 6)
Note: Incidence is based on the number of patients with at least one amputation, and not the total number of amputation events. A patient’s follow-up is calculated from Day 1 to the first amputation event date. Some patients had more than one amputation.
Volume Depletion
In a trial of adults with type 2 diabetes mellitus and moderate renal impairment (Trial 5), adverse reactions related to volume depletion (e.g., dehydration, dizziness, dizziness postural, vertigo, vertigo positional, presyncope, hypotension, and orthostatic hypotension) were reported in 3.9% and 8.9% of patients treated with placebo and BRENZAVVY, respectively.
Genital Mycotic Infections
In a pool of three placebo-controlled clinical trials (12-week dose ranging trial and Trials 1 and 2), the incidence of female genital mycotic infections occurred in 0% and 5.6% of females treated with placebo and BRENZAVVY, respectively (see Table 1). In the same pool of trials, male genital mycotic infections occurred in 1.4% and 2.2% of males treated with placebo and BRENZAVVY, respectively (see Table 1). In a trial that enrolled adults with type 2 diabetes mellitus and moderate renal impairment (Trial 5), 0% and 9.2% of female patients treated with placebo and BRENZAVVY, respectively, had a genital mycotic infection.
In a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), 2.8% and 9.0% of patients treated with placebo and BRENZAVVY, respectively, had at least one event of genital mycotic infection. In the same trial, genital mycotic infections that caused drug discontinuation were reported in 0% and 1.2% of patients treated with placebo and BRENZAVVY, respectively. Balanoposthitis was reported in 0% and 2.9% of male patients, and phimosis was reported in 0.3% and 0.5% of male patients treated with placebo and BRENZAVVY, respectively. Patients treated with BRENZAVVY with events of phimosis typically underwent circumcision.
Fractures
In a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), the incidence rates of serious fractures, including events of hip and femur fracture, were 1.4 and 5.4 events per 1,000 patient-years of follow-up in the placebo and BRENZAVVY groups, respectively. The imbalance in serious fractures was observed within the first 6 months of therapy and remained through the end of the trial.
Hypoglycemia
The incidence of hypoglycemia by trial is shown in Table 3.
In a trial of adults with type 2 diabetes mellitus and moderate renal impairment (Trial 5), adverse reactions related to volume depletion (e.g., dehydration, dizziness, dizziness postural, vertigo, vertigo positional, presyncope, hypotension, and orthostatic hypotension) were reported in 3.9% and 8.9% of patients treated with placebo and BRENZAVVY, respectively.
Genital Mycotic Infections
In a pool of three placebo-controlled clinical trials (12-week dose ranging trial and Trials 1 and 2), the incidence of female genital mycotic infections occurred in 0% and 5.6% of females treated with placebo and BRENZAVVY, respectively (see Table 1). In the same pool of trials, male genital mycotic infections occurred in 1.4% and 2.2% of males treated with placebo and BRENZAVVY, respectively (see Table 1). In a trial that enrolled adults with type 2 diabetes mellitus and moderate renal impairment (Trial 5), 0% and 9.2% of female patients treated with placebo and BRENZAVVY, respectively, had a genital mycotic infection.
In a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), 2.8% and 9.0% of patients treated with placebo and BRENZAVVY, respectively, had at least one event of genital mycotic infection. In the same trial, genital mycotic infections that caused drug discontinuation were reported in 0% and 1.2% of patients treated with placebo and BRENZAVVY, respectively. Balanoposthitis was reported in 0% and 2.9% of male patients, and phimosis was reported in 0.3% and 0.5% of male patients treated with placebo and BRENZAVVY, respectively. Patients treated with BRENZAVVY with events of phimosis typically underwent circumcision.
Fractures
In a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), the incidence rates of serious fractures, including events of hip and femur fracture, were 1.4 and 5.4 events per 1,000 patient-years of follow-up in the placebo and BRENZAVVY groups, respectively. The imbalance in serious fractures was observed within the first 6 months of therapy and remained through the end of the trial.
Hypoglycemia
The incidence of hypoglycemia by trial is shown in Table 3.
Table 3. Incidence of Overall
* Overall hypoglycemia: plasma or capillary glucose of less than 54 mg/dL.
† Severe hypoglycemia: patient required assistance, lost consciousness, or experienced a seizure (irrespective of blood glucose concentration).
β No restrictions were placed on background antihyperglycemic therapy (aside from treatment with another SGLT2 inhibitor) and approximately 50% of patients used insulin and/or an insulin secretagogue at baseline.
Rash and Dermatitis
In the clinical program of BRENZAVVY, one event of rash and one event of dermatitis was confirmed to be attributable to BRENZAVVY exposure by withdrawal and rechallenge. The rash and dermatitis events occurred on day 37 and day 3 of exposure to BRENZAVVY, respectively. In a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), 3.4% and 5.4% of patients experienced at least one event of rash with placebo and BRENZAVVY, respectively.
† Severe hypoglycemia: patient required assistance, lost consciousness, or experienced a seizure (irrespective of blood glucose concentration).
β No restrictions were placed on background antihyperglycemic therapy (aside from treatment with another SGLT2 inhibitor) and approximately 50% of patients used insulin and/or an insulin secretagogue at baseline.
Rash and Dermatitis
In the clinical program of BRENZAVVY, one event of rash and one event of dermatitis was confirmed to be attributable to BRENZAVVY exposure by withdrawal and rechallenge. The rash and dermatitis events occurred on day 37 and day 3 of exposure to BRENZAVVY, respectively. In a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), 3.4% and 5.4% of patients experienced at least one event of rash with placebo and BRENZAVVY, respectively.
SepsisBRENZAVVY was associated with an increased risk of sepsis/septic shock events, including events that may have caused or contributed to death, in a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6). Sepsis events occurred in 2 (0.4%) and 14 (1.2%) of placebo-treated patients and BRENZAVVY-treated patients in the trial, respectively. Of these, 1 sepsis event among placebo-treated patients and 3 sepsis events among BRENZAVVY-treated patients were related to urinary tract infections.
Laboratory AbnormalitiesChanges in Serum Creatinine and eGFRInitiation of BRENZAVVY causes an increase in serum creatinine and decrease in eGFR within weeks of starting therapy that stabilizes by week 6 to 12. In a trial enrolling adults with type 2 diabetes mellitus and moderate renal impairment (Trial 5), a mean change in serum creatinine of 0.0 mg/dL and a decrease in eGFR of 0.1 mL/min/1.73 m2 was observed in the placebo group as compared to a mean increase in serum creatinine of 0.1 mg/dL and a mean decrease in eGFR of 4.6 mL/min/1.73 m2 with BRENZAVVY, within the first 6 weeks of treatment. In a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), an initial decrease in eGFR was seen within weeks of starting therapy (eGFR changes from baseline to week 12 of 0 and -3.1 mL/min/1.73 m2 in the placebo and BRENZAVVY arms, respectively). Acute hemodynamic changes may play a role in the early renal function changes observed with BRENZAVVY since they are reversed after treatment discontinuation.
Increases in Low-Density Lipoprotein Cholesterol (LDL-C)
In a pool of two placebo-controlled clinical trials (Trials 1 and 2), mean LDL-C decreased by 3.8 mg/dL (3.7%) in patients treated with placebo (N = 195) and increased by 1.7 mg/dL (1.6%) in patients treated with BRENZAVVY (N = 247) at week 24. In a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), LDL-C increased by 3 mg/dL (3.2%) and 3 mg/dL (4.1%) with placebo and BRENZAVVY treatment, respectively, at Week 24.
In a pool of two placebo-controlled clinical trials (Trials 1 and 2), mean LDL-C decreased by 3.8 mg/dL (3.7%) in patients treated with placebo (N = 195) and increased by 1.7 mg/dL (1.6%) in patients treated with BRENZAVVY (N = 247) at week 24. In a trial that enrolled adults with type 2 diabetes mellitus who had either established CVD or were at increased risk for CVD (Trial 6), LDL-C increased by 3 mg/dL (3.2%) and 3 mg/dL (4.1%) with placebo and BRENZAVVY treatment, respectively, at Week 24.
Increases in Hemoglobin and Hematocrit
In a pool of two placebo-controlled trials (Trials 1 and 2), mean changes from baseline to Week 24 in hemoglobin were -0.3 g/dL (-2.1%) with placebo and 0.4 g/dL (2.9%) with BRENZAVVY. In the same pool, mean changes from baseline to Week 24 in hematocrit were -0.6% with placebo and 1.3% with BRENZAVVY 20 mg. Fewer patients had > 2 g/dL increases in hemoglobin from baseline for placebo (0.5%) compared to BRENZAVVY (4.9%). Increases in hemoglobin > 3 g/dL from baseline were observed in 0% of placebo-treated patients compared to 0.7% of BRENZAVVY-treated patients.
In a pool of two placebo-controlled trials (Trials 1 and 2), mean changes from baseline to Week 24 in hemoglobin were -0.3 g/dL (-2.1%) with placebo and 0.4 g/dL (2.9%) with BRENZAVVY. In the same pool, mean changes from baseline to Week 24 in hematocrit were -0.6% with placebo and 1.3% with BRENZAVVY 20 mg. Fewer patients had > 2 g/dL increases in hemoglobin from baseline for placebo (0.5%) compared to BRENZAVVY (4.9%). Increases in hemoglobin > 3 g/dL from baseline were observed in 0% of placebo-treated patients compared to 0.7% of BRENZAVVY-treated patients.
5DRUG INTERACTIONS
See
Table 4. Clinically Significant Interactions with BRENZAVVY
6OVERDOSAGE
In the event of an overdose of BRENZAVVY, consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations. Employ the usual supportive measures as dictated by the patient’s clinical status. Removal of bexagliflozin by hemodialysis has not been studied.
7DESCRIPTION
BRENZAVVY tablets for oral use contain bexagliflozin, an SGLT2 inhibitor.
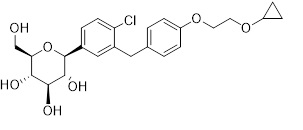
Bexagliflozin is a white/off-white to pale yellow powder. It is very slightly soluble in water and freely soluble in methanol, acetone, ethylene glycol, and propylene glycol. It is slightly soluble in heptane, cyclohexane, and toluene. Crystalline bexagliflozin is not hygroscopic.
Each film-coated tablet contains 20 mg of bexagliflozin and the inactive ingredients colloidal silicon dioxide, glyceryl dibehenate, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene oxide, and poloxamer 188. In addition, the film coating ingredient, Opadry® II Blue 85F99153, contains the inactive ingredients FD&C Blue #1/Brilliant Blue FCF Aluminum Lake, FD&C Blue #2/Indigo Carmine Aluminum Lake, macrogol 3350, partially hydrolyzed polyvinyl alcohol, talc, and titanium dioxide.
8HOW SUPPLIED/STORAGE AND HANDLING
BRENZAVVY 20 mg tablets are blue, caplet-shaped, biconvex, bevel-edged, film-coated debossed with “2” and inverted “2” on one side.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Lower Limb AmputationInform patients of the potential for an increased risk of amputations with BRENZAVVY. Counsel patients on the importance of routine preventive foot care. Instruct patients to monitor for any new pain or tenderness, sores or ulcers, or infections involving the leg or foot and to seek medical advice immediately if such signs or symptoms develop [see Warnings and Precautions (5.2)].
Volume DepletionInform patients that symptomatic hypotension may occur with BRENZAVVY and advise them to contact their healthcare provider if they experience such symptoms. Inform patients that dehydration may increase the risk of hypotension, and to maintain adequate fluid intake. [see Warnings and Precautions (5.3)].
Genitourinary Infections, including Urosepsis, Pyelonephritis, Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene), and Genital Mycotic InfectionsCounsel patients that genitourinary infections may occur when taking BRENZAVVY and may become serious. Educate them on the symptoms and advise them to seek medical advice if symptoms occur. Specifically, advise patients to promptly seek medical attention if they develop pain or tenderness, redness, or swelling of the genitals or the area from the genitals back to the rectum, along with a fever above 100.4°F or malaise. [see Warnings and Precautions (5.4)].
Hypoglycemia with Concomitant Use with Insulin and Insulin SecretagoguesInform patients that the incidence of hypoglycemia may increase when BRENZAVVY is added to insulin and/or an insulin secretagogue. Educate patients or caregivers on the signs and symptoms of hypoglycemia [see Warnings and Precautions (5.5)].
PregnancyAdvise pregnant patients and females of reproductive potential of the potential risk to a fetus with treatment with BRENZAVVY. Instruct patients to inform their healthcare provider if pregnant or planning to become pregnant [see Use in Specific Populations (8.1)].
LactationAdvise patients that breastfeeding is not recommended during treatment with BRENZAVVY [see Use in Specific Populations (8.2)].
Laboratory TestsInform patients that their urine will test positive for glucose while taking BRENZAVVY due to its mechanism of action. [see Drug Interactions (
Missed DoseInstruct patients to take BRENZAVVY only as prescribed. If a dose is missed, it should be taken as soon as possible. Advise patients not to double their next dose.
Distributed by:
10MEDGUIDE SECTION
This Medication Guide has been approved by the U.S. Food and Drug Administration Issued: 11/2025
11PACKAGE LABEL.PRINCIPAL DISPLAY PANEL