Generic Name
Metoclopramide
Brand Names
Gimoti, Reglan
FDA approval date: September 30, 1990
Classification: Dopamine-2 Receptor Antagonist
Form: Injection, Spray, Tablet, Solution
What is Gimoti (Metoclopramide)?
Diabetic Gastroparesis Metoclopramide Injection, USP is indicated for the relief of symptoms associated with acute and recurrent diabetic gastric stasis. The Prevention of Nausea and Vomiting Associated with Emetogenic Cancer Chemotherapy Metoclopramide Injection, USP is indicated for the prophylaxis of vomiting associated with emetogenic cancer chemotherapy. The Prevention of Postoperative Nausea and Vomiting Metoclopramide Injection, USP is indicated for the prophylaxis of postoperative nausea and vomiting in those circumstances where nasogastric suction is undesirable. Small Bowel Intubation Metoclopramide Injection, USP may be used to facilitate small bowel intubation in adults and pediatric patients in whom the tube does not pass the pylorus with conventional maneuvers. Radiological Examination Metoclopramide Injection, USP may be used to stimulate gastric emptying and intestinal transit of barium in cases where delayed emptying interferes with radiological examination of the stomach and/or small intestine.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
GIMOTI (METOCLOPRAMIDE HYDROCHLORIDE)
1INDICATIONS AND USAGE
GIMOTI is indicated for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis.
2DOSAGE FORMS AND STRENGTHS
Nasal Spray: 15 mg of metoclopramide in each 70 microliter spray. GIMOTI is an aqueous solution supplied in an amber glass bottle fitted with a metered spray pump attachment.
3CONTRAINDICATIONS
GIMOTI is contraindicated:
- In patients with a history of tardive dyskinesia (TD) or a dystonic reaction to metoclopramide
- When stimulation of gastrointestinal motility might be dangerous (e.g., in the presence of gastrointestinal hemorrhage, mechanical obstruction, or perforation).
- In patients with pheochromocytoma or other catecholamine-releasing paragangliomas. Metoclopramide may cause a hypertensive/pheochromocytoma crisis, probably due to release of catecholamines from the tumor
- In patients with epilepsy. Metoclopramide may increase the frequency and severity of seizures
- In patients with hypersensitivity to metoclopramide. Reactions have included laryngeal and glossal angioedema and bronchospasm
4ADVERSE REACTIONS
The following adverse reactions are described, or described in greater detail, in other sections of the labeling:
- Tardive dyskinesia
- Other extrapyramidal effects
- Neuroleptic malignant syndrome
- Depression
- Hypertension
- Fluid retention
- Hyperprolactinemia
- Effects on the ability to drive and operate machinery
The following adverse reactions have been identified from clinical studies or postmarketing reports of metoclopramide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The safety of GIMOTI was evaluated in clinical trials of patients with gastroparesis and established in clinical trials of oral metoclopramide.
5OVERDOSAGE
Manifestations of metoclopramide overdosage included drowsiness, disorientation, extrapyramidal reactions, other adverse reactions associated with metoclopramide use (including, e.g., methemoglobinemia), and sometimes death. Neuroleptic malignant syndrome (NMS) has been reported in association with metoclopramide overdose and concomitant treatment with another drug associated with NMS
There are no specific antidotes for metoclopramide overdosage. If over-exposure occurs, call your Poison Control Center at 1-800-222-1222 for current information on the management of poisoning or overdosage
Methemoglobinemia can be reversed by the intravenous administration of methylene blue. However, methylene blue may cause hemolytic anemia in patients with G6PD deficiency, which may be fatal.
Hemodialysis and continuous ambulatory peritoneal dialysis do not remove significant amounts of metoclopramide.
6DESCRIPTION
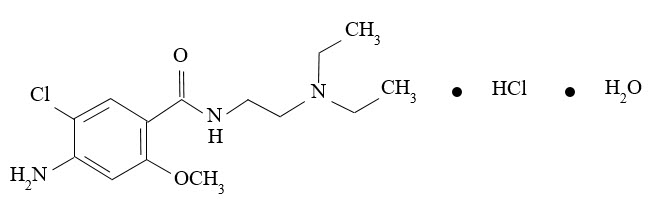
Metoclopramide hydrochloride, the active ingredient in GIMOTI, is a dopamine-2 receptor antagonist. Metoclopramide hydrochloride is a white, crystalline, odorless substance, freely soluble in water. Its chemical name is 4-amino5-chloro-N-[2-(diethylamino)ethyl]-2-methoxy benzamide monohydrochloride monohydrate.
The molecular formula is C
GIMOTI (metoclopramide) nasal spray is for nasal administration. The product is supplied as an aqueous solution with a pH of 5.5 ± 0.5 in a 10 mL amber glass vial fitted with a metered spray pump attachment. Each unit contains 9.8 mL.
Each 70 microliter spray contains 15 mg metoclopramide, equivalent to 17.73 mg of metoclopramide hydrochloride. Inactive ingredients consist of benzalkonium chloride, citric acid monohydrate, edetate disodium dihydrate, purified water, sodium citrate dihydrate, and sorbitol.
7CLINICAL STUDIES
The effectiveness of GIMOTI has been established based on studies of oral metoclopramide for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis.
8HOW SUPPLIED/STORAGE AND HANDLING
GIMOTI (metoclopramide) nasal spray is supplied as a solution of metoclopramide in a 10 mL Type 1 amber glass bottle fitted with a metered spray pump attachment, a protective cap, and a safety clip. Each box of GIMOTI (NDC 72089-307-15) contains 1 bottle, with FDA-approved Patient Labeling (see
Each actuation delivers 15 mg of metoclopramide. Each bottle contains 9.8 mL which is sufficient for 4 weeks of 4 times a day use.
9PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
10Instructions for Use
GIMOTI™ (jye-MOH-tee) (phonetic spelling)
Read this Instructions for Use before you start using GIMOTI nasal spray and each time you get a refill. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Important information:
- GIMOTI is for use in your nose only.
- The GIMOTI nasal spray bottle has enough medicine for 4 weeks of treatment if taken 4 times each day.
- 1 dose is 1 spray in 1 nostril.
- Your GIMOTI nasal spray bottle must be primed:
- Store the GIMOTI nasal spray at room temperature between 68°F to 77°F (20°C to 25°C).
- Throw away (discard) the GIMOTI nasal spray 4 weeks after opening even if the bottle contains unused medicine.
- Keep out of the reach of children.
Parts of your GIMOTI bottle
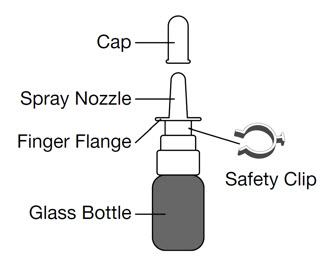
Steps to use GIMOTI
- Uncap the GIMOTI nasal spray bottle
- Remove the cap from the spray nozzle by pulling straight up.
- Prime the GIMOTI nasal spray bottle
- Remove the safety clip from the spray pump.
- Place your index finger and middle finger on each side of the finger flange and your thumb on the bottom of the glass bottle.
- Turn the spray nozzle away from your face, keeping the bottle upright.
- Press down firmly and release 10 times on the finger flange until a spray appears from the spray nozzle. You may not see a spray the first few times you press down. After pressing down and releasing a spray 10 times, the GIMOTI nasal spray will be primed and ready to use.
If you are not able to press and release 10 sprays from the GIMOTI nasal spray, call your healthcare provider or pharmacist.
The
- Using the GIMOTI nasal spray
- Place the spray nozzle tip under one of your nostrils. Tilt your head slightly forward so the tip of the spray nozzle is aimed away from the center of your nose.
- Close the other nostril with your other index finger. Move the spray pump up so the tip of the nozzle is in your nostril.
- Press down firmly on the finger flange until it stops moving to deliver a full dose.
- Release the finger flange and breathe in gently through the open nostril.
- Remove the spray pump nozzle tip from your nostril and breathe out slowly through your mouth.
- After use, wipe the spray nozzle with a clean tissue. Place the cap on the spray nozzle by pushing straight down. Place the safety clip back onto the spray pump.
Cleaning the spray pump nozzle
If the spray pump nozzle becomes clogged, remove it for cleaning by grasping the base of the spray nozzle and pulling up.
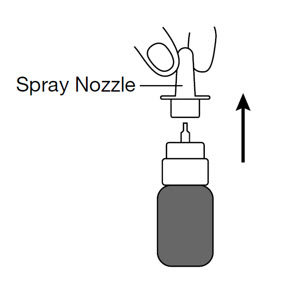
Soak the spray nozzle in warm water and rinse.
Dry the spray nozzle at room temperature. When the spray nozzle is dry, place the dry spray nozzle back on the GIMOTI bottle.
Disposal instructions
The used GIMOTI nasal spray may be thrown away (discarded) in the household trash.
Manufactured for Evoke Pharma, Inc. by Patheon, a Division of Thermo Fisher.
GIMOTI is a trademark of Evoke Pharma.
For more information, go to www.evokepharma.com or call 1-833-4-GIMOTI (1-833-444-6684).
These Instructions for Use have been approved by the U.S. Food and Drug Administration
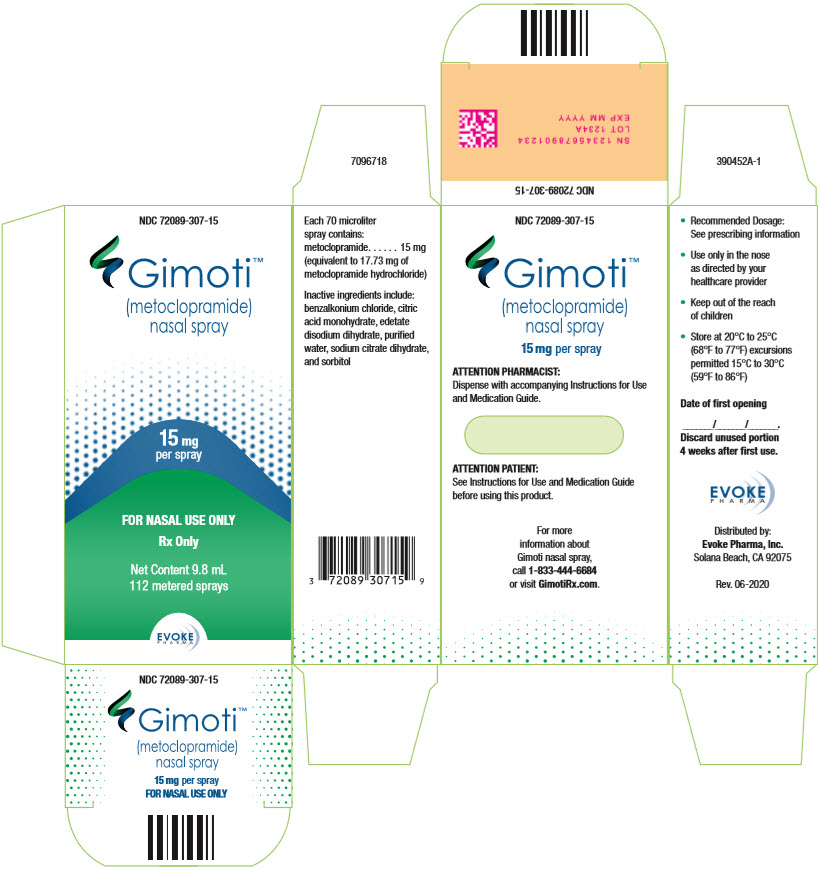
11PRINCIPAL DISPLAY PANEL - 9.8 mL Bottle Carton
NDC 72089-307-15
Gimoti™
15 mg
FOR NASAL USE ONLY
Rx Only
Net Content 9.8 mL
EVOKE