Brand Name
FROVA
Generic Name
Frovatriptan
View Brand Information FDA approval date: November 08, 2001
Classification: Serotonin-1b and Serotonin-1d Receptor Agonist
Form: Tablet
What is FROVA (Frovatriptan)?
Frovatriptan succinate tablets are indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use Use only if a clear diagnosis of migraine has been established. If a patient has no response for the first migraine attack treated with frovatriptan succinate tablets, reconsider the diagnosis of migraine before frovatriptan succinate tablets are administered to treat any subsequent attacks. Frovatriptan succinate tablets are not indicated for the prevention of migraine attacks. Safety and effectiveness of frovatriptan succinate tablets have not been established for cluster headache. Frovatriptan succinate is a serotonin receptor agonist indicated for the acute treatment of migraine with or without aura in adults Limitations of Use Use only after a clear diagnosis of migraine has been established Not indicated for the prophylactic therapy of migraine Not indicated for the treatment of cluster headache
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
FROVA (frovatriptan succinate)
1INDICATIONS AND USAGE
FROVA is indicated for the acute treatment of migraine with or without aura in adults.
Limitations of Use
Use only if a clear diagnosis of migraine has been established. If a patient has no response for the first migraine attack treated with FROVA, reconsider the diagnosis of migraine before FROVA is administered to treat any subsequent attacks. FROVA is not indicated for the prevention of migraine attacks. Safety and effectiveness of FROVA have not been established for cluster headache.
2DOSAGE AND ADMINISTRATION
Dosing Information
The recommended dose is a single tablet of FROVA (frovatriptan 2.5 mg) taken orally with fluids.
If the migraine recurs after initial relief, a second tablet may be taken, providing there is an interval of at least 2 hours between doses. The total daily dose of FROVA should not exceed 3 tablets (3 x 2.5 mg per 24-hour period).
There is no evidence that a second dose of FROVA is effective in patients who do not respond to a first dose of the drug for the same headache.
The safety of treating an average of more than 4 migraine attacks in a 30-day period has not been established.
3DOSAGE FORMS AND STRENGTHS
2.5 mg Tablets: Round, white, film-coated tablets debossed with 2.5 on one side and "E" on the other side.
4CONTRAINDICATIONS
FROVA is contraindicated in patients with:
Ischemic coronary artery disease (CAD) (e.g., angina pectoris, history of myocardial infarction, or documented silent ischemia), or coronary artery vasospasm, including Prinzmetal’s angina Wolff-Parkinson-White Syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders History of stroke, transient ischemic attack (TIA), or history of hemiplegic or basilar migraine because these patients are at a higher risk of stroke Peripheral vascular disease Ischemic bowel disease Uncontrolled hypertension Recent use (i.e., within 24 hours) of another 5-HT Hypersensitivity to frovatriptan succinate (angioedema and anaphylaxis seen)
5ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in other sections of the labeling:
- Myocardial Ischemia, Myocardial Infarction, and Prinzmetal’s Angina
- Arrhythmias
- Chest, Throat, Neck, and/or Jaw Pain/Tightness/Pressure
- Cerebrovascular Events
- Other Vasospasm Reactions
- Medication Overuse Headache
- Serotonin Syndrome
- Increases in Blood Pressure
- Hypersensitivity Reactions
5.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
FROVA was evaluated in four randomized, double-blind, placebo-controlled, short-term trials. These trials involved 2392 patients (1554 on FROVA 2.5 mg and 838 on placebo). In these short-term trials, patients were predominately female (88%) and Caucasian (94%) with a mean age of 42 years (range 18 to 69).The treatment-emergent adverse events that occurred most frequently following administration of FROVA 2.5 mg (i.e.,in at least 2% of patients), and at an incidence ≥1% greater than with placebo, were dizziness, paresthesia, headache, dry mouth, fatigue, flushing, hot or cold sensation, dyspepsia, skeletal pain, and chest pain. In a long-term, open-label study where 496 patients were allowed to treat multiple migraine attacks with FROVA 2.5 mg for up to 1 year, 5% of patients (n=26) discontinued due to treatment-emergent adverse events.
Table 1 lists treatment-emergent adverse events reported within 48 hours of drug administration that occurred with FROVA 2.5 mg at an incidence of ≥2% and more often than on placebo, in the four placebo-controlled trials. The events cited reflect experience gained under closely monitored conditions of clinical trials in a highly selected patient population. In actual clinical practice or in other clinical trials, these incidence estimates may not apply, as the conditions of use, reporting behavior, and the kinds of patients treated may differ.
Table 1:
The incidence of adverse events in clinical trials did not increase when up to 3 doses were used within 24 hours. The incidence of adverse events in placebo-controlled clinical trials was not affected by gender, age, or concomitant medications commonly used by migraine patients. There were insufficient data to assess the impact of race on the incidence of adverse events.
Other Events Observed in Association with the Administration of FROVA
The incidence of frequently reported adverse events in four placebo-controlled trials is presented below. Events are further classified within body system categories. Frequent adverse events are those occurring in at least 1/100 patients.
Central and peripheral nervous system: dysesthesia and hypoesthesia.
Gastrointestinal: vomiting, abdominal pain and diarrhea.
Body as a whole: pain.
Psychiatric: insomnia and anxiety.
Respiratory: sinusitis and rhinitis.
Vision disorders: vision abnormal.
Skin and appendages: sweating increased.
Hearing and vestibular disorders: tinnitus.
Heart rate and rhythm: palpitation.
5.2Postmarketing Experience
The following adverse reactions were identified during post approval use of FROVA. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Central and peripheral nervous system: Seizure.
6OVERDOSAGE
The elimination half-life of frovatriptan is 26 hours [
7DESCRIPTION
FROVA (frovatriptan succinate) tablets contain frovatriptan succinate, a selective 5-hydroxy-tryptamine1 (5-HT
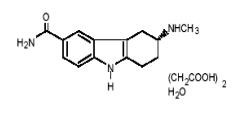
The empirical formula is C
Each FROVA tablet for oral administration contains 3.91 mg frovatriptan succinate, equivalent to 2.5 mg of frovatriptan base. Each tablet also contains the inactive ingredients lactose NF, microcrystalline cellulose NF, colloidal silicon dioxide NF, sodium starch glycolate NF, magnesium stearate NF, hypromellose USP, polyethylene glycol 3000 USP, triacetin USP, and titanium dioxide USP.
8CLINICAL STUDIES
The efficacy of FROVA in the acute treatment of migraine headaches was demonstrated in four randomized, double-blind, placebo-controlled, short-term outpatient trials. In these trials, patients received doses of frovatriptan from 0.5 mg to 40 mg. In these controlled short-term trials, patients were predominately female (88%) and Caucasian (94%) with a mean age of 42 years (range 18 to 69). Patients were instructed to treat a moderate to severe headache. Headache response, defined as a reduction in headache severity from moderate or severe pain to mild or no pain, was assessed for up to 24 hours after dosing. The associated symptoms nausea, vomiting, photophobia, and phonophobia were also assessed. Maintenance of response was assessed for up to 24 hours post dose. In two of the trials a second dose of FROVA was provided after the initial treatment, to treat recurrence of the headache within 24 hours. Other medication, excluding other 5-HT
In all four placebo-controlled trials, the percentage of patients achieving a headache response 2 hours after treatment was significantly greater for those taking FROVA 2.5 mg compared to those taking placebo (Table 2).
Lower doses of frovatriptan (1 mg or 0.5 mg) were not effective at 2 hours. Higher doses (5 mg to 40 mg) of frovatriptan showed no added benefit over 2.5 mg but did cause a greater incidence of adverse events.
Table 2:
aITT observed data, excludes patients who had missing data or were asleep.
*p<0.05.
**p<0.001 in comparison with placebo.
The estimated probability of achieving an initial headache response by 2 hours following treatment is depicted in Figure 1.
Figure 1:
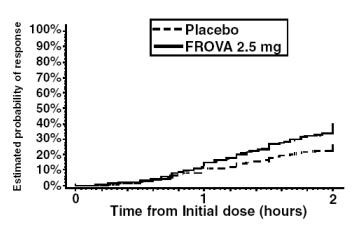
Figure 1 shows a Kaplan-Meier plot of the probability over time of obtaining headache response (no or mild pain) following treatment with FROVA 2.5 mg or placebo. The probabilities displayed are based on pooled data from the four placebo-controlled trials described in Table 2. Patients who did not achieve a response were censored at 24 hours.
In patients with migraine-associated nausea, photophobia, and phonophobia at baseline there was a decreased incidence of these symptoms in FROVA treated patients compared to placebo.
The estimated probability of patients taking a second dose or other medication for their migraine over the 24 hours following the initial dose of study treatment is summarized in Figure 2.
Figure 2:
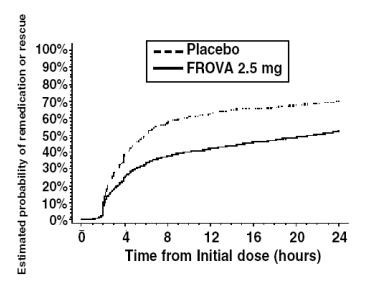
Figure 2 is a Kaplan-Meier plot showing the probability of patients taking a second dose or other medication for migraine over the 24 hours following the initial dose of study medication based on the data from the four placebo-controlled trials described in Table 2. The plot includes those patients who had a response to the initial dose and those who did not. The protocols did not permit remedication within 2 hours of the initial dose.
Efficacy was unaffected by a history of aura; gender; age, or concomitant medications commonly used by migraine patients.
9HOW SUPPLIED/STORAGE AND HANDLING
FROVA tablets, containing 2.5 mg of frovatriptan (base) as the succinate salt, are available as round, white, film-coated tablets debossed with 2.5 on one side and “E” on the other side. The tablets are available in:
Blister card of 9 tablets, 1 blister card per carton (NDC 63481-025-09)
Store FROVA tablets at controlled room temperature, 25ºC (77ºF) excursions permitted to 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature]. Protect from moisture.
10PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Myocardial Ischemia and/or Infarction, Prinzmetal’s Angina, Other Vasospastic Reactions, and Cerebrovascular Events
Inform patients that FROVA may cause serious cardiovascular adverse reactions such as myocardial infarction or stroke, which may result in hospitalization and even death. Although serious cardiovascular reactions can occur without warning symptoms, instruct patients to be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and instruct them to ask for medical advice when observing any indicative sign or symptoms. Instruct patients to seek medical advice if they have symptoms of other vasospastic reactions
Hypersensitivity Reactions
Inform patients that anaphylactic reactions have occurred in patients receiving FROVA. Inform patients that such reactions can be life threatening or fatal and to seek immediate medical attention if they have anaphylactic symptoms. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens
Medication Overuse Headache
Inform patients that use of drugs to treat acute migraines for 10 or more days per month may lead to an exacerbation of headache, and encourage patients to record headache frequency and drug use (e.g., by keeping a headache diary)
Serotonin Syndrome
Inform patients about the risk of serotonin syndrome with the use of FROVA or other triptans, particularly during combined use with SSRIs, SNRIs, TCAs, and MAO inhibitors
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant during treatment or plan to become pregnant
Lactation
Inform patients to notify their healthcare provider if they are breastfeeding or plan to breastfeed
Manufactured for:
Endo USA
Malvern, PA 19355
Manufactured by:
Almac Pharma Services Limited
Craigavon, BT63 5UA, UK
FROVA is a registered trademark of Vernalis Development Limited.
U.S. Patent Nos 5,962,501, 5,827,871, 5,637,611 and 5,464,864 and 5,616,603.
© 2024 Endo, Inc. or one of its affiliates.
Revision: July 2024
11PATIENT LABELING
This Patient Information has been approved by the U.S. Food and Drug Administration
Revised: July 2024
