Generic Name
Brinzolamide
Brand Names
Azopt, Simbrinza
FDA approval date: April 30, 1998
Classification: Carbonic Anhydrase Inhibitor
Form: Suspension
What is Azopt (Brinzolamide)?
AZOPT is a carbonic anhydrase inhibitor indicated in the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma. AZOPT is a carbonic anhydrase inhibitor indicated for the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Azopt (brinzolamide)
1INDICATIONS AND USAGE
AZOPT is a carbonic anhydrase inhibitor indicated in the treatment of elevated intraocular pressure (IOP) in patients with ocular hypertension or open-angle glaucoma.
2DOSAGE AND ADMINISTRATION
The recommended dose is one drop of AZOPT in the affected eye(s) 3 times daily. Shake well before use. AZOPT may be used concomitantly with other topical ophthalmic drug products to lower IOP. If more than one topical ophthalmic drug is being used, the drugs should be administered at least 10 minutes apart.
3DOSAGE FORMS AND STRENGTHS
Ophthalmic suspension containing brinzolamide 1% (10 mg/mL).
4CONTRAINDICATIONS
AZOPT is contraindicated in patients who are hypersensitive to any component of this product.
5OVERDOSAGE
Although no human data are available, electrolyte imbalance, development of an acidotic state, and possible nervous system effects may occur following oral administration of an overdose. Serum electrolyte levels (particularly potassium) and blood pH levels should be monitored.
6DESCRIPTION
AZOPT contains a carbonic anhydrase inhibitor formulated for multidose topical ophthalmic use. Brinzolamide is described chemically as: (R)-(+)-4-Ethylamino-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno [3,2-e]-1,2-thiazine-6-sulfonamide-1,1- dioxide. Its empirical formula is C
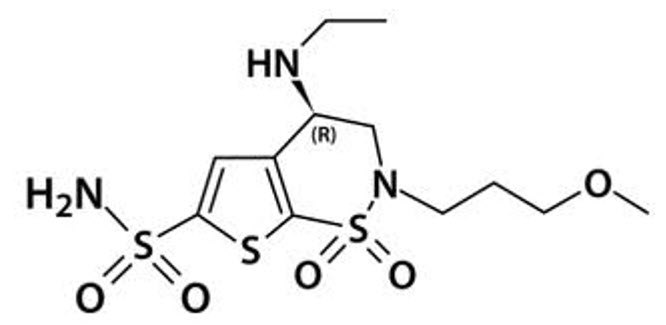
Brinzolamide has a molecular weight of 383.5 g/mol and a melting point of about 131°C. It is a white powder, which is insoluble in water, very soluble in methanol and soluble in ethanol.
AZOPT is supplied as a sterile, aqueous suspension of brinzolamide which has been formulated to be readily suspended and slow settling, following shaking. It has a pH of approximately 7.5 and an osmolality of 300 mOsm/kg.
Each mL of AZOPT contains:
7CLINICAL STUDIES
In two, 3-month clinical studies, AZOPT dosed 3 times per day in patients with elevated IOP, produced significant reductions in IOPs (4 mmHg to 5 mmHg). These IOP reductions are equivalent to the reductions observed with TRUSOPT* (dorzolamide hydrochloride ophthalmic solution) 2% dosed 3 times per day in the same studies.
In 2 clinical studies in patients with elevated IOP, AZOPT was associated with less stinging and burning upon instillation than TRUSOPT* 2%.
8HOW SUPPLIED/STORAGE AND HANDLING
AZOPT (brinzolamide ophthalmic suspension) 1% is supplied in plastic dispensers with a controlled dispensing-tip as follows:
10 mL NDC 66758-085-70
15 mL NDC 66758-085-85
Storage and Handling
Store AZOPT at 4°C to 30°C (39°F to 86°F). Shake well before use. After opening, AZOPT can be used until the expiration date on the bottle.
9PATIENT COUNSELING INFORMATION
Sulfonamide Hypersensitivity Reactions
Advise patients that if serious or unusual ocular or systemic reactions or signs of hypersensitivity occur, they should discontinue the use of the product and consult their physician immediately
Temporary Blurred Vision
Vision may be temporarily blurred following dosing with AZOPT. Advise patients to exercise care in operating machinery or driving a motor vehicle.
Avoiding Contamination of the Product
Instruct patients to avoid allowing the tip of the dispensing container to contact the eye or surrounding structures or other surfaces, since the product can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
Intercurrent Ocular Conditions
Advise patients that if they have ocular surgery or develop an intercurrent ocular condition (e.g., trauma or infection), they should immediately seek their physician's advice concerning the continued use of the present multi-dose container.
Concomitant Topical Ocular Therapy
If more than one topical ophthalmic drug is being used, the drugs should be administered at least ten minutes apart.
Contact Lens Wear
The preservative in AZOPT, benzalkonium chloride, may be absorbed by soft contact lenses. Contact lenses should be removed during instillation of AZOPT, but may be reinserted 15 minutes after instillation.
*TRUSOPT is a registered trademark of Merck & Co., Inc.
Manufactured by
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 for
Sandoz Inc., Princeton, NJ 08540
300064739-1123
10PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 66758-085-70
Azopt®
(brinzolamide
ophthalmic
suspension) 1%
10 mL Sterile
Sandoz

11PRINCIPAL DISPLAY PANEL
NDC 66758-085-70
Azopt
(brinzolamide
10 mL Sterile
Sandoz
