Brand Name
Xdemvy
Generic Name
Lotilaner
View Brand Information FDA approval date: August 14, 2023
Classification: Ectoparasiticide
Form: Solution
What is Xdemvy (Lotilaner)?
XDEMVY is indicated for the treatment of Demodex blepharitis. XDEMVY is an ectoparasiticide indicated for the treatment of Demodex blepharitis.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
XDEMVY (Lotilaner ophthalmic solution)
1INDICATIONS AND USAGE
XDEMVY is indicated for the treatment of Demodex blepharitis.
2DOSAGE AND ADMINISTRATION
Instill one drop of XDEMVY in each eye twice daily (approximately 12 hours apart) for 6 weeks. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart. If one dose is missed, treatment should continue with the next scheduled dose.
3DOSAGE FORMS AND STRENGTHS
XDEMVY (lotilaner ophthalmic solution) is provided as an ophthalmic solution containing lotilaner 0.25% (2.5 mg/mL).
4CONTRAINDICATIONS
None.
5DESCRIPTION
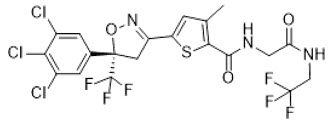
Lotilaner is a member of the isoxazoline family of compounds. Its chemical name is 2-Thiophenecarboxamide, 5-[(5S)-4,5-dihydro-5-(3,4,5-trichlorophenyl)-5-(trifluoromethyl)-3-isoxazolyl]-3-methyl-N-[2-oxo-2-[(2,2,2-trifluoroethyl)amino]ethyl]-2-thiophenecarboxamide. The molecular formula is C
XDEMVY is a sterile, preserved, multi-dose, slightly yellowish, slightly opalescent, topical ophthalmic solution containing lotilaner, 0.25% as the active ingredient. It is preserved with potassium sorbate and contains the following additional inactive ingredients: edetate disodium, hydroxypropyl methylcellulose (HPMC), polyoxyl 35 castor oil, glycerin, dibasic sodium phosphate, monobasic sodium phosphate, and water for injection.
6CLINICAL STUDIES
The safety and efficacy of XDEMVY for the treatment of Demodex blepharitis was evaluated in a total of 833 patients (415 of which received XDEMVY) in two 6-week, randomized, multicenter, double-masked, vehicle-controlled studies (Saturn-1 and Saturn-2). Patients with Demodex blepharitis were randomized to either XDEMVY or Vehicle at a 1:1 ratio dosed twice daily in each eye.
Efficacy was demonstrated by improvement in lids (reduction of collarettes to no more than 2 collarettes per upper lid) in each study (Saturn-1 and Saturn-2) (see
Figure 1. Saturn-1: Proportion of patients with 2 or less collarettes for the upper eyelid
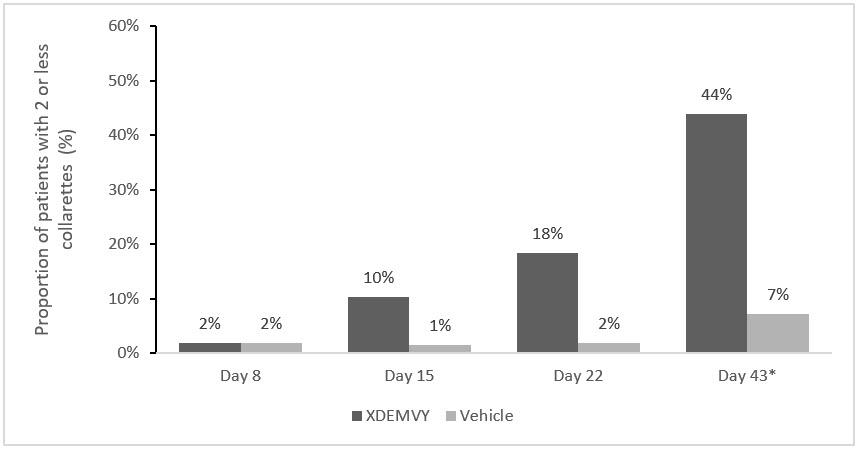
*Day 43 Primary Endpoint; XDEMVY N=209, Vehicle N=204, p-value <0.01
Figure 2. Saturn-2: Proportion of patients with 2 or less collarettes for the upper eyelid
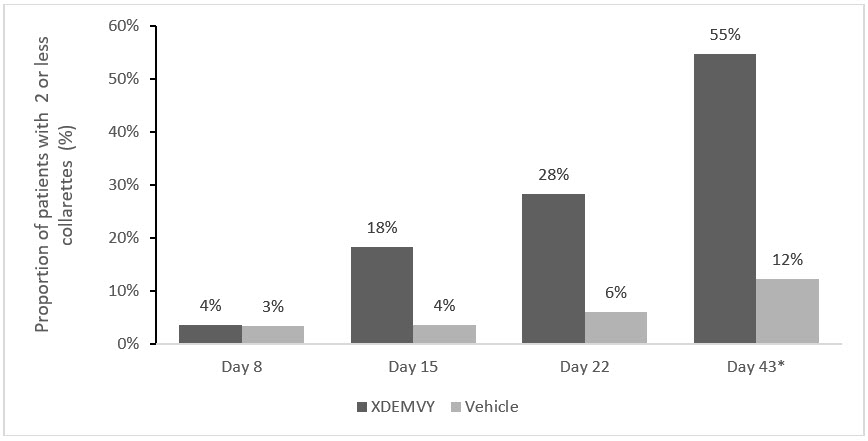
*Day 43 Primary Endpoint; XDEMVY N=193, Vehicle N=200, p-value <0.01
The endpoints of mite eradication (mite density of 0 mites/lash) and erythema cure (Grade 0) of XDEMVY vs. Vehicle demonstrated statistically significant improvement at Day 43 across both Saturn-1 (Table 1) and Saturn-2 (Table 2) studies.
7HOW SUPPLIED/STORAGE AND HANDLING
XDEMVY is supplied as a sterile ophthalmic solution in a white, opaque, low-density polyethylene (LDPE) multiple-dose bottle with a LDPE dropper tip and tan high-density polyethylene (HDPE) cap.
8PRINCIPAL DISPLAY PANEL - 1.5 mL Bottle Carton
Rx Only
xdemvy™
Physician Sample
For Topical Application in the Eye
NDC 81942-125-99
9PRINCIPAL DISPLAY PANEL - 10 mL Bottle Carton
Rx Only
xdemvy™
For Topical Application in the Eye
NDC 81942-125-01
