Rytelo
What is Rytelo (Imetelstat)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This phase I trial tests the safety, side effects, and best dose of imetelstat in combination with fludarabine and cytarabine in treating patients with acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) or juvenile myelomonocytic leukemia (JMML) that has not responded to previous treatment (refractory) or that has come back after a period of improvement (recurrent). Imetelstat may stop t...
Summary: The purpose of the study is to identify the recommended Part 2 dose (R2PD) of imetelstat sodium in combination with ruxolitinib in participants with myelofibrosis (MF) in Part 1, and to evaluate the safety and clinical activity of the R2PD of imetelstat sodium in combination with ruxolitinib or other Janus Kinase (JAK) inhibitors in participants with MF in Part 2.
Related Latest Advances
Brand Information
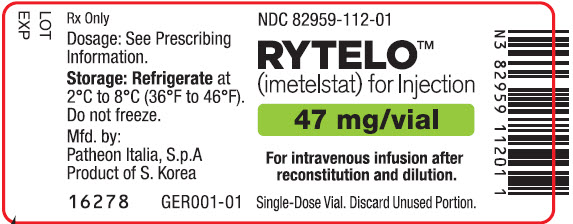
- For injection: 47 mg of imetelstat supplied as a white to off-white or slightly yellow lyophilized powder in a single-dose vial for reconstitution.
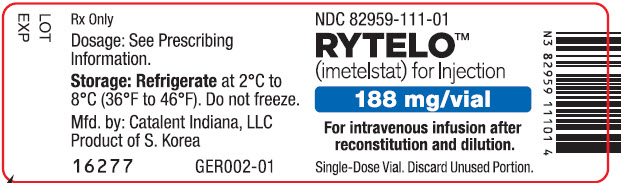
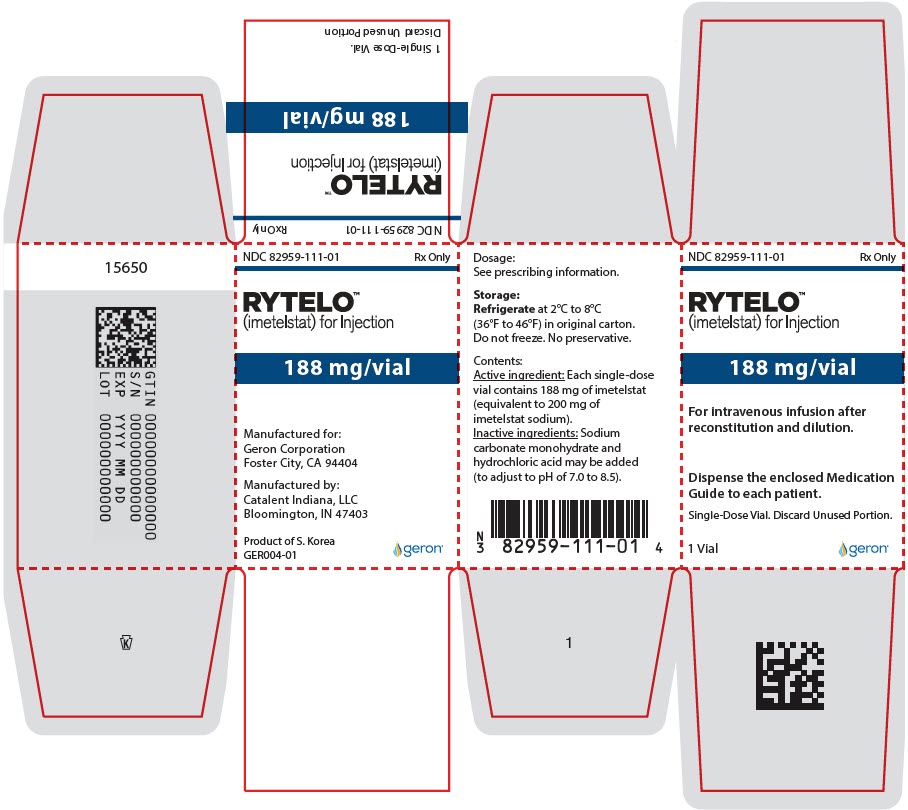
- For injection: 188 mg of imetelstat supplied as a white to off-white or slightly yellow lyophilized powder in a single-dose vial for reconstitution.
- Thrombocytopenia
- Neutropenia [see
- Infusion-Related Reactions