Abrilada
What is Abrilada (Adalimumab-Afzb)?
Living with chronic inflammatory diseases like rheumatoid arthritis, Crohn’s disease, or psoriasis can make everyday life unpredictable. The pain, swelling, and fatigue can affect everything from mobility to emotional well-being. Abrilada (adalimumab-afzb) is a medication designed to help control these immune-related conditions, offering relief and improving quality of life for many people.
Abrilada is a biosimilar to Humira (adalimumab), one of the most widely used biologic drugs in the world. It belongs to a class of medications known as tumor necrosis factor (TNF) blockers, which work by targeting a key protein in the body’s immune system that causes inflammation. Approved by the U.S. Food and Drug Administration (FDA) in 2019 and launched in 2023, Abrilada provides patients with a more affordable alternative to Humira while maintaining the same safety, efficacy, and quality standards.
As a long-term maintenance therapy, Abrilada helps patients gain better control of chronic inflammatory diseases that have not responded well to traditional treatments, helping restore comfort and stability to daily life.
What does Abrilada do?
Abrilada is used to reduce inflammation and prevent the immune system from attacking healthy tissues. It treats several autoimmune and inflammatory diseases, including:
- Rheumatoid arthritis (RA): easing joint pain, swelling, and stiffness
- Psoriatic arthritis: improving joint mobility and skin symptoms
- Ankylosing spondylitis: relieving back pain and improving spinal flexibility
- Crohn’s disease and ulcerative colitis: reducing abdominal pain, diarrhea, and inflammation in the digestive tract
- Plaque psoriasis: decreasing thick, scaly patches of skin
- Juvenile idiopathic arthritis: helping children with autoimmune joint inflammation
Patients who take Abrilada often report less pain, reduced inflammation, and improved physical function over time. Clinical studies have demonstrated that Abrilada delivers comparable results to Humira, meaning it performs just as effectively in managing disease symptoms and preventing progression (FDA, 2023).
For many, this medication helps restore mobility, reduces fatigue, and allows them to reengage with daily activities that once felt impossible.
How does Abrilada work?
Abrilada contains adalimumab-afzb, a monoclonal antibody that specifically targets tumor necrosis factor-alpha (TNF-α), a naturally occurring protein in the immune system. In people with autoimmune diseases, TNF-α is overproduced, triggering inflammation that damages joints, skin, and other tissues.
By binding to TNF-α, Abrilada blocks its inflammatory action, preventing it from attaching to its receptors and activating the immune response. This reduces swelling, pain, and tissue damage throughout the body.
In simple terms, Abrilada helps “calm down” an overactive immune system. Clinically, this mechanism is vital because it not only relieves symptoms but also helps slow or stop long-term joint and tissue damage, preserving mobility and function.
The drug does not act as a general immune suppressant; rather, it targets a specific pathway, allowing the immune system to continue protecting the body against infections while minimizing harmful inflammation.
Abrilada side effects
As with all biologic therapies, Abrilada can cause side effects, though many are mild and manageable. It’s important to recognize what’s typical and when to contact a healthcare provider.
Common side effects may include:
- Mild pain, redness, or swelling at the injection site
- Headache or fatigue
- Upper respiratory infections (such as colds or sinus infections)
- Nausea
Serious side effects (less common):
- Signs of infection such as fever, persistent cough, or shortness of breath
- Nervous system problems, including vision changes or numbness
- Heart failure symptoms (shortness of breath, swelling in legs)
- Blood disorders (unusual bruising or bleeding)
- Allergic reactions, including rash or facial swelling
Abrilada impacts the immune system, increasing infection risk (TB, fungal, viral). Patients are screened for TB and hepatitis B before treatment.
Avoid Abrilada with active infections, severe heart failure, or adalimumab allergies. Discuss risks with a doctor if you have a history of cancer, demyelinating disease, or chronic infections.
Seek immediate medical attention for severe rash, breathing issues, or persistent fever. Regular doctor visits help manage side effects.
Abrilada dosage
Abrilada is a subcutaneous injection, usually given every two weeks, though the schedule varies by condition. It comes in a pre-filled syringe or autoinjector for convenient self-administration at home, typically in the abdomen or thigh, rotating sites to prevent irritation.
Doctors might suggest regular blood tests to check liver function, blood counts, and infection markers, ensuring the medication’s continued effectiveness and safety. Abrilada is generally safe for older adults and those with chronic liver or kidney disease, though closer monitoring may be necessary. Patients should continue their medication as prescribed, even if symptoms improve, to prevent inflammation from returning.
Does Abrilada have a generic version?
Abrilada is a biosimilar, which means it is a highly similar version of a biologic drug, in this case, Humira. Biosimilars are not considered traditional generics because they are made from living cells, but they are equally effective, safe, and high-quality as their reference biologic.
The FDA has approved Abrilada as a biosimilar to Humira, confirming no meaningful clinical differences in safety, purity, or potency. It is approved for all the same medical indications as Humira. While there’s no generic Abrilada, other Humira biosimilars like Amjevita and Hyrimoz are available, providing affordable alternatives with similar benefits and ensuring the same results as Humira in symptom relief, safety, and quality.
Conclusion
Abrilada (adalimumab-afzb) represents a significant advancement in access to biologic therapy for people living with chronic inflammatory and autoimmune diseases. By precisely targeting the body’s TNF-α pathway, it helps control inflammation, reduce pain, and prevent long-term damage, all while improving daily function and quality of life.
An FDA-approved biosimilar to Humira, Abrilada offers a trusted, cost-effective alternative with the same therapeutic benefits. Side effects are possible but generally manageable with monitoring and communication with a healthcare team. Consistent use and medical supervision can help patients achieve stability, comfort, and confidence, making long-term disease management more hopeful.
References
- U.S. Food and Drug Administration (FDA). (2023). Abrilada (adalimumab-afzb) prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Adalimumab (injection route) description and precautions. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Adalimumab injection: Drug information. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). TNF inhibitors and autoimmune disease management. Retrieved from https://www.nih.gov
Approved To Treat
Top Global Experts
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
- Active tuberculosis (TB), including reactivation of latent TB. Patients with TB have frequently presented with disseminated or extrapulmonary disease. Test patients for latent TB before ABRILADA use and during therapy. Initiate treatment for latent TB prior to ABRILADA use.
- Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Consider empiric anti-fungal therapy in patients at risk for invasive fungal infections who develop severe systemic illness.
- Bacterial, viral and other infections due to opportunistic pathogens, including Legionella and Listeria.
- Prefilled Pen (ABRILADA Pen)
Injection: 40 mg/0.8 mL in a single-dose pen. - Prefilled Syringe
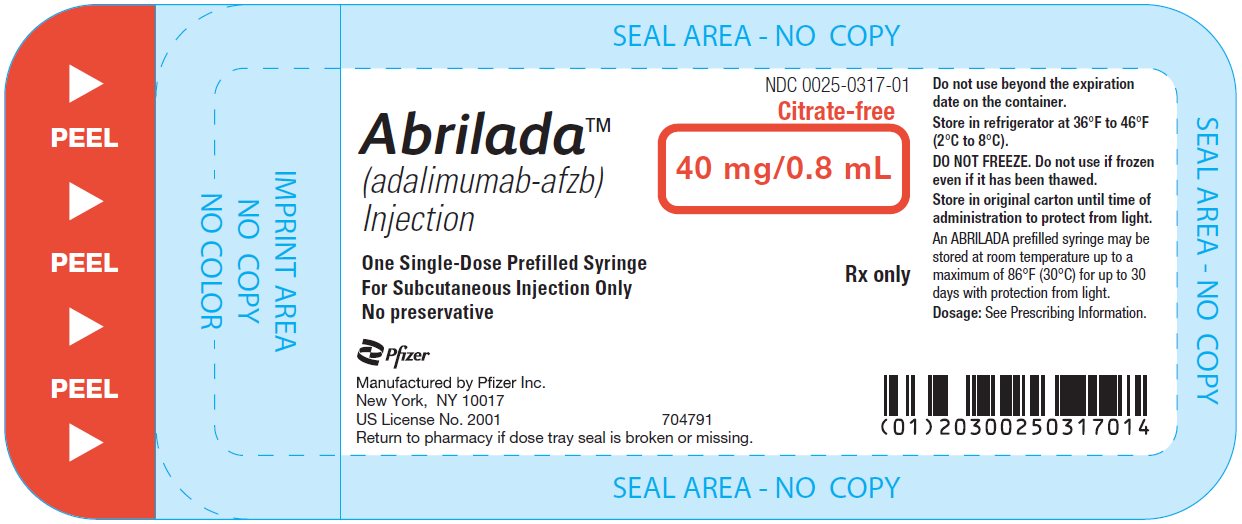
Injection: 40 mg/0.8 mL in a single-dose prefilled glass syringe.
Injection: 20 mg/0.4 mL in a single-dose prefilled glass syringe.
Injection: 10 mg/0.2 mL in a single-dose prefilled glass syringe. - Single-Dose Institutional Use Vial
Injection: 40 mg/0.8 mL in a single-dose glass vial for institutional use only.
- Serious Infections
- Malignancies
- Hypersensitivity Reactions
- Hepatitis B Virus Reactivation
- Neurologic Reactions
- Hematological Reactions
- Heart Failure
- Autoimmunity
- Gastrointestinal disorders: Diverticulitis, large bowel perforations including perforations associated with diverticulitis and appendiceal perforations associated with appendicitis, pancreatitis
- General disorders and administration site conditions: Pyrexia
- Hepato-biliary disorders: Liver failure, hepatitis, autoimmune hepatitis
- Immune system disorders: Sarcoidosis
- Neoplasms benign, malignant and unspecified (including cysts and polyps): Merkel Cell Carcinoma (neuroendocrine carcinoma of the skin)
- Nervous system disorders: Demyelinating disorders (e.g., optic neuritis, Guillain-Barré syndrome), cerebrovascular accident
- Respiratory disorders: Interstitial lung disease, including pulmonary fibrosis, pulmonary embolism
- Skin reactions: Stevens Johnson Syndrome, cutaneous vasculitis, erythema multiforme, new or worsening psoriasis (all sub-types including pustular and palmoplantar), alopecia, lichenoid skin reaction
- Vascular disorders: Systemic vasculitis, deep vein thrombosis
- National Cancer Institute. Surveillance, Epidemiology, and End Results Database (SEER) Program. SEER Incidence Crude Rates, 17 Registries, 2000–2007.
- ABRILADA Pen Carton - 40 mg/0.8 mL (One Count)
ABRILADA (adalimumab-afzb) injection is supplied in a carton containing two alcohol preps and a single-dose pen. The single-dose pen contains a 1 mL prefilled glass syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.8 mL of ABRILADA.
The NDC numbers are 0069-0325-01 and 0025-0325-01. - ABRILADA Pen Carton - 40 mg/0.8 mL (Two Counts)
ABRILADA (adalimumab-afzb) injection is supplied in a carton containing two alcohol preps and two single-dose pens. Each single-dose pen contains a 1 mL prefilled glass syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.8 mL of ABRILADA.
The NDC numbers are 0069-0325-02 and 0025-0325-02. - Prefilled Syringe Carton - 40 mg/0.8 mL (One Count)
ABRILADA is supplied in a carton containing two alcohol preps and one dose tray. The dose tray consists of a single-dose, 1 mL prefilled glass syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.8 mL of ABRILADA.
The NDC numbers are 0069-0328-01 and 0025-0328-01. - Prefilled Syringe Carton - 40 mg/0.8 mL (Two Counts)
ABRILADA is supplied in a carton containing two alcohol preps and two dose trays. Each dose tray consists of a single-dose, 1 mL prefilled glass syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.8 mL of ABRILADA.
The NDC numbers are 0069-0328-02 and 0025-0328-02. - Prefilled Syringe Carton - 20 mg/0.4 mL (Two Counts)
ABRILADA is supplied in a carton containing two alcohol preps and two dose trays. Each dose tray consists of a single-dose, 1 mL prefilled glass syringe with a fixed thin wall, ½ inch needle, providing 20 mg/0.4 mL of ABRILADA.
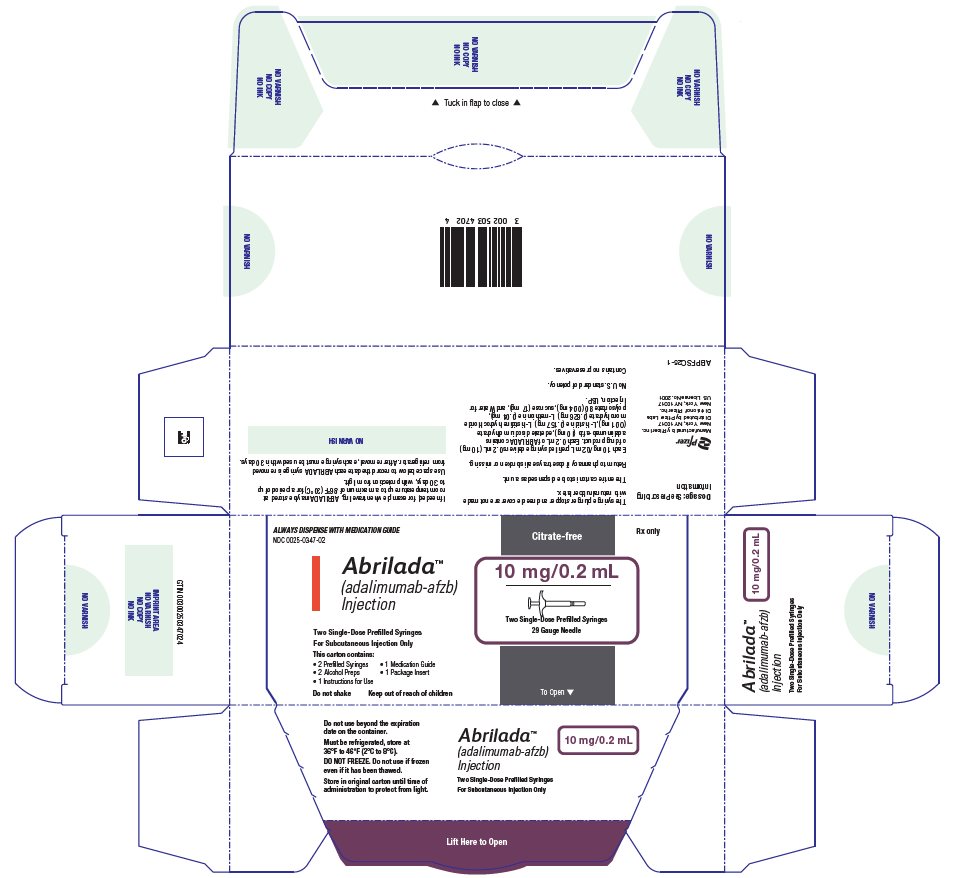
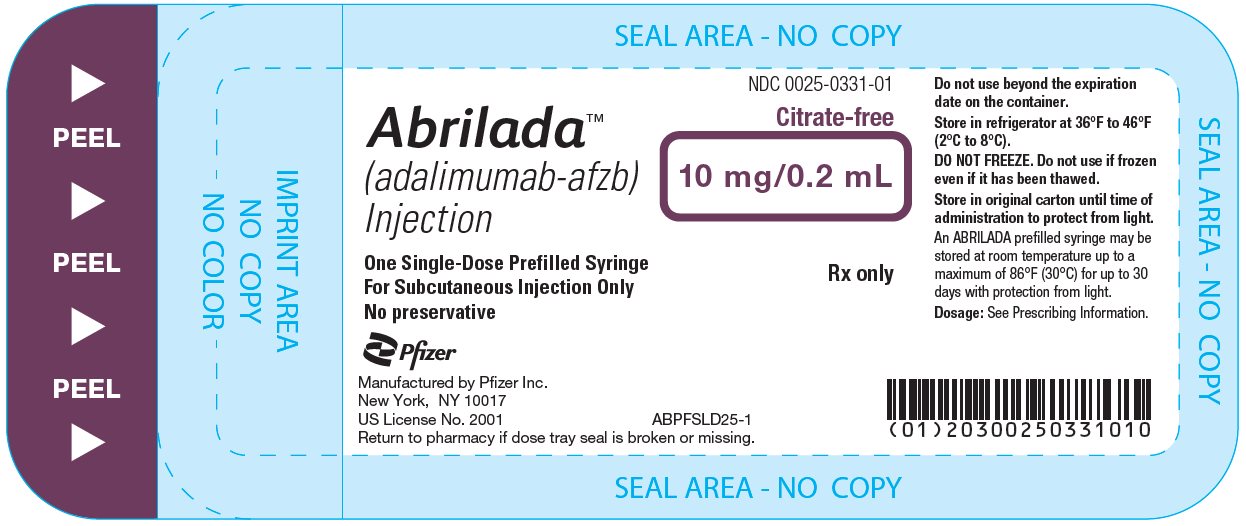
The NDC numbers are 0069-0333-02 and 0025-0333-02. - Prefilled Syringe Carton - 10 mg/0.2 mL (Two Counts)
ABRILADA is supplied in a carton containing two alcohol preps and two dose trays. Each dose tray consists of a single-dose, 1 mL prefilled glass syringe with a fixed thin wall, ½ inch needle, providing 10 mg/0.2 mL of ABRILADA.
The NDC numbers are 0069-0347-02 and 0025-0347-02. - Single-Dose Institutional Use Vial Carton - 40 mg/0.8 mL
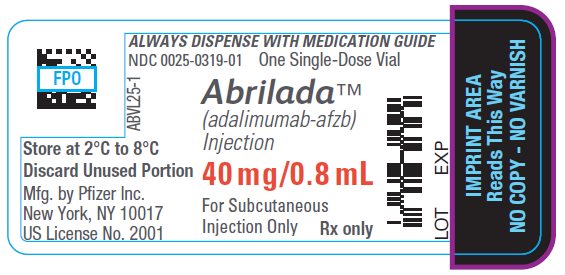
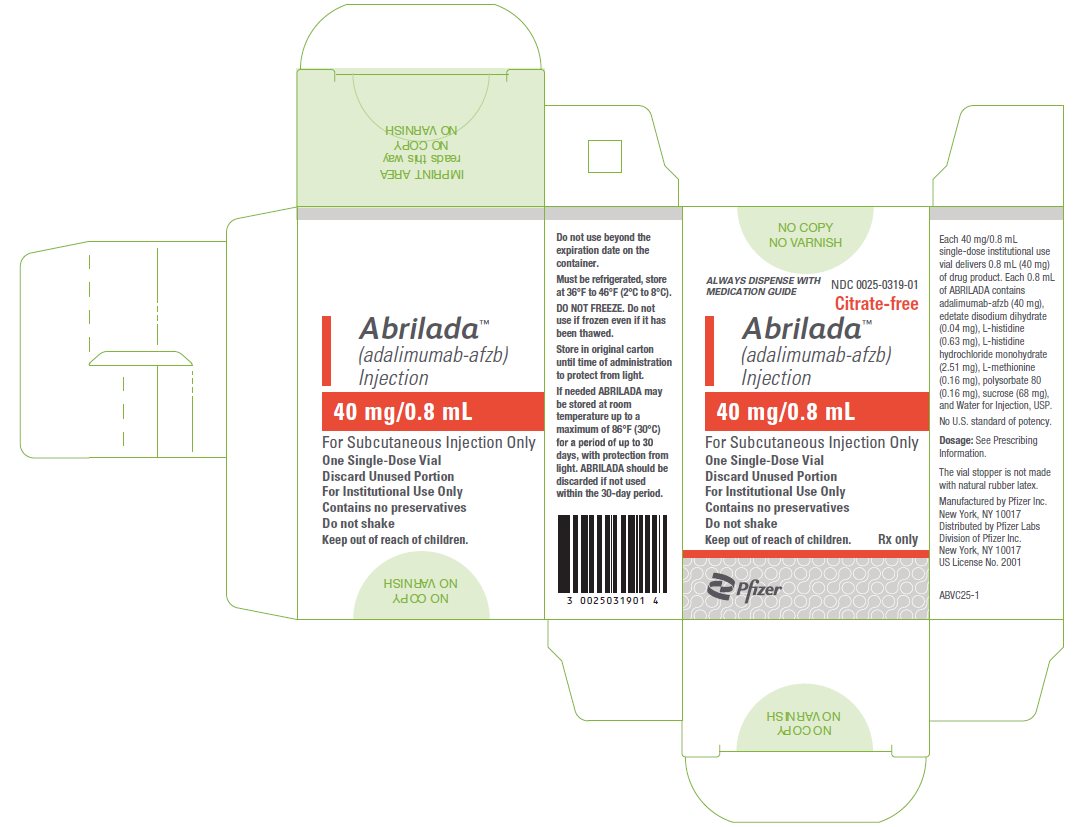
ABRILADA is supplied for institutional use only in a carton containing a single-dose, glass vial, providing 40 mg/0.8 mL of ABRILADA. The vial stopper is not made with natural rubber latex.
The NDC numbers are 0069-0319-01 and 0025-0319-01.
(adalimumab-afzb)
40 mg/0.8 mL
Single-dose Prefilled Pen
Injection, for subcutaneous (under the skin) use
- Store your ABRILADA pen in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Store ABRILADA pen in the original carton until use to protect from light.
- Do not freeze ABRILADA. Do not use ABRILADA if frozen, even if it has been thawed.
- Refrigerated ABRILADA may be used until the expiration date printed on the ABRILADA carton or pen. Do not use ABRILADA after the expiration date.
- If needed, for example when you are traveling, you may also store ABRILADA at room temperature up to 86°F (30°C) for up to 30 days. Store ABRILADA in the original carton until use to protect it from light.
- Throw away ABRILADA if it has been kept at room temperature and not been used within 30 days.
- Record the date you first remove ABRILADA from the refrigerator in the spaces provided on the ABRILADA pen carton.
- Do not store ABRILADA in extreme heat or cold.
- Do not use the pen if the liquid is cloudy, discolored, or has flakes or particles in it.
- You will need the following supplies for each injection of ABRILADA. Find a clean, flat surface to place the supplies on.
- Remove the ABRILADA carton from the refrigerator.
- Make sure the name ABRILADA appears on the carton and prefilled pen label.
- Take out 1 ABRILADA pen and the alcohol swab. Keep your pen out of direct sunlight. Put the original carton with any unused pens back in the refrigerator.
- Do not use your pen if:
- your pen or the carton containing the pen has been dropped
- it has been frozen or thawed
- it has been kept in direct sunlight
- it appears to be damaged
- the seals on a new carton are broken
- it has been out of the refrigerator for more than 30 days
- the expiration date has passed
- the liquid is cloudy, discolored or has flakes or particles
- For a more comfortable injection, you may leave your pen at room temperature for 15 to 30 minutes before your injection.
- Do not warm ABRILADA in any other way (for example, do not warm it in a microwave or in hot water).
- Do not shake your pen. Shaking can damage your medicine.
- Wash your hands with soap and water, and dry completely.
- Do not remove the cap until you are ready to inject.
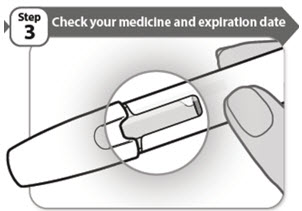
- Look carefully at your medicine in the window.
- Make sure the medicine in the pen is clear and colorless to very light brown and free from flakes or particles.
- It is normal to see one or more air bubbles in the window.
- Check the expiration date on the pen label. The location of the expiration date on the pen label is shown below.
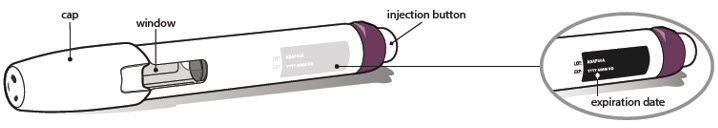
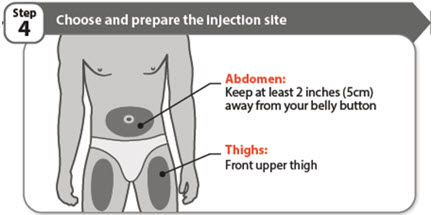
- Choose a different injection site each time you give an injection:
- Do not inject into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- If you have psoriasis, do not inject directly into any raised, thick, red, or scaly skin patches or lesions on your skin.
- Do not inject through your clothes.
- Wipe the injection site with the alcohol swab.
- Allow the injection site to dry.
- Do not touch this area again before giving the injection.
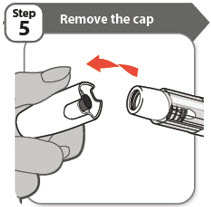
- Twist and pull off the cap.
- Throw the cap away into a sharps disposal container. You will not need it again.
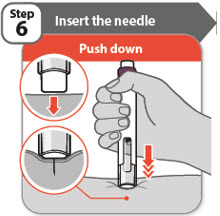
- Push your pen firmly against the skin at 90 degrees, as shown in the diagram.
Note: The needle goes into the skin as you push your pen down. You will only be able to press down the injection button in Step 7 when you are pushing down firmly enough. - Keep your pen pushed against the skin until Step 9.
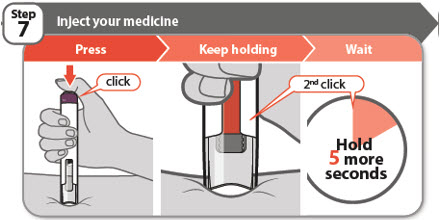
- Press the injection button all the way down and you will hear a click. The click means the start of the injection.
- Keep holding your pen firmly against the skin while the orange bar moves across the window. You will hear a 2nd click.
- Wait for at least 5 more seconds after the 2nd click to make sure you get the full dose of medicine.
Note: If you cannot press down the injection button, it is because you are not pushing the pen down firmly enough at the injection site. See the Question and Answer section on the right side of this Instructions for Use for more information on what to do if the injection button does not press down.
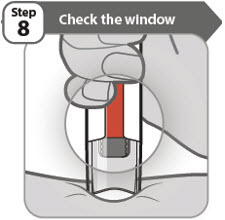
- You should see an orange bar in the window.
- Do not remove your pen until you have waited at least 5 seconds after the 2
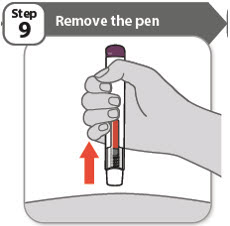
- Remove your pen from the skin.
Note: After you remove your pen from the skin, the needle will be automatically covered. - If the window has not turned orange, this means you have not received a full dose. Call your healthcare provider or pharmacist right away.
- Do not inject another dose.
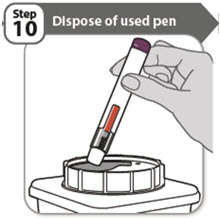
- Put your used pen in a FDA-cleared sharps disposal container right away after use.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
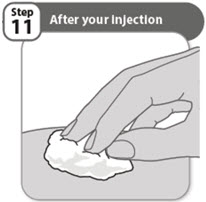
- Look closely at your injection site. If there is blood, use a clean cotton ball or gauze pad to press lightly on the injection area for a few seconds.
- Do not rub the injection site.
Note: Store any unused pens in the refrigerator in the original carton.

(adalimumab-afzb)
10 mg/0.2 mL, 20 mg/0.4 mL, 40 mg/0.8 mL
Single-dose Prefilled Syringe
- Store your ABRILADA prefilled syringe in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Store ABRILADA prefilled syringe in the original carton until use to protect from light.
- Do not freeze ABRILADA. Do not use ABRILADA if frozen, even if it has been thawed.
- Refrigerated ABRILADA may be used until the expiration date printed on the ABRILADA carton or prefilled syringe. Do not use ABRILADA after the expiration date.
- If needed, for example when you are traveling, you may also store ABRILADA at room temperature up to 86°F (30°C) for up to 30 days. Store ABRILADA in the original carton until use to protect it from light.
- Throw away ABRILADA if it has been kept at room temperature and not been used within 30 days.
- Record the date you first remove ABRILADA from the refrigerator in the spaces provided on the ABRILADA prefilled syringe carton.
- Do not store ABRILADA in extreme heat or cold.
- Do not use a prefilled syringe if the liquid is cloudy, discolored, or has flakes or particles in it.
- Do not drop or crush ABRILADA. The prefilled syringe is glass.
- You will need the following supplies for each injection of ABRILADA. Find a clean, flat surface to place the supplies on.
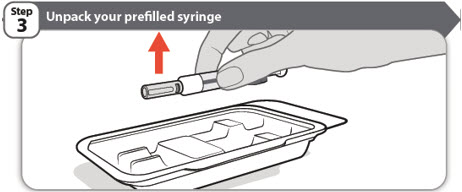
- Remove ABRILADA carton from the refrigerator.
- Open the carton and take out the tray containing your prefilled syringe.
- Make sure the name ABRILADA appears on the dose tray and prefilled syringe label.
- Check your carton and tray.
- Wash your hands with soap and water, and dry completely.
- Peel back the paper seal on the tray.
- Remove 1 prefilled syringe from the tray and put the original carton with any unused prefilled syringes back in the refrigerator.
- Do not use your syringe if:
- it appears to be damaged
- It has been kept in direct sunlight
- liquid is cloudy, discolored, or has flakes or particles
- Do not shake your syringe. Shaking can damage your medicine.
- For a more comfortable injection, leave the prefilled syringe at room temperature for 15 to 30 minutes before your injection.
- Do not warm ABRILADA in any other way (for example, do not warm it in a microwave or in hot water).
- Do not remove the needle cover from your prefilled syringe until you are ready to inject.
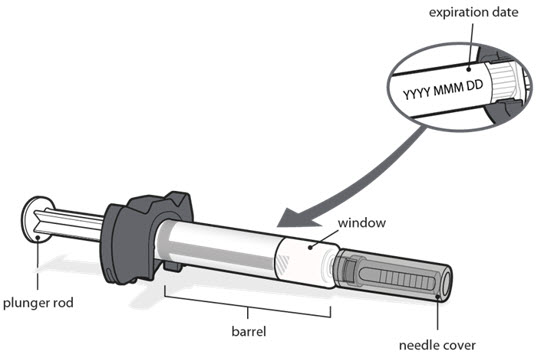
Always hold the prefilled syringe by the barrel to prevent damage.
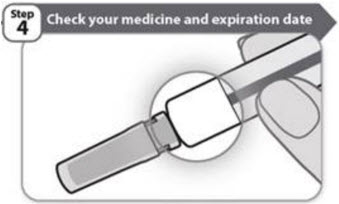
- Look carefully at your medicine in the window.
- Make sure the medicine in the prefilled syringe is clear and colorless to very light brown and free from flakes or particles.
- It is normal to see one or more air bubbles in the window.
- Check the expiration date on the prefilled syringe label as shown in the figure in Step 1. Do not use the prefilled syringe if the expiration date has passed.
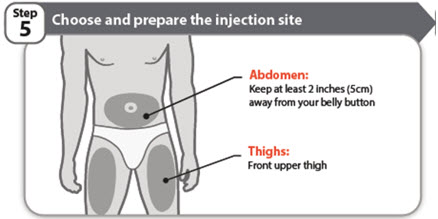
- Choose a different injection site each time you give an injection.
- Do not inject into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- If you have psoriasis, do not inject directly into any raised, thick, red, or scaly skin patches or lesions on your skin.
- Do not inject through your clothes.
- Wipe the injection site with the alcohol swab.
- Allow the injection site to dry.
- Do not touch this area again before giving the injection.
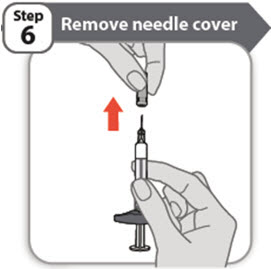
- Hold the prefilled syringe by the barrel. Carefully pull the needle cover straight off and away from your body when you are ready to inject.
- It is normal to see a drop of liquid at the end of the needle.
- Throw the needle cover away into a sharps disposal container.
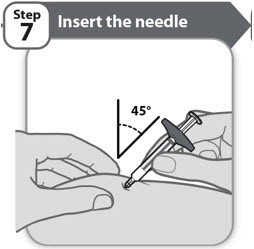
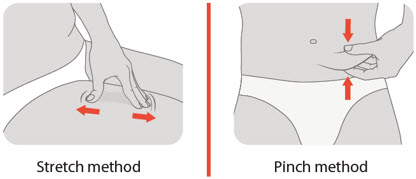
- Gently pinch up a fold of skin in the cleaned injection site area.
- Insert the needle to its full depth into the skin, at
- After the needle is inserted, release the pinched skin.
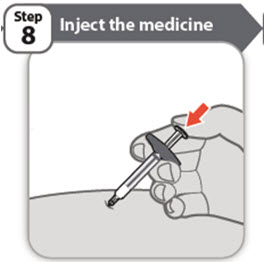
- Using slow and constant pressure, push the plunger rod all the way down until the barrel is empty.
- Pull the needle out of the skin at the same angle at which it entered.
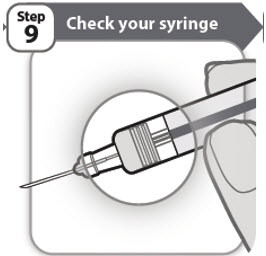
- Check that your medicine has completely emptied from your prefilled syringe. If the gray stopper is not in the position shown, you may not have injected all of your medicine. Contact your healthcare provider right away.
- Never re-insert the needle.
- Never put the cap back on the needle.
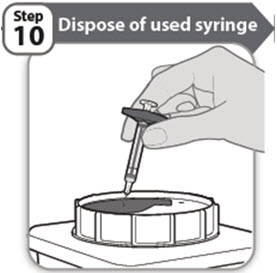
- Put your used syringe in a FDA-cleared sharps disposal container right away after use.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Look closely at your injection site. If there is blood, use a clean cotton ball or gauze pad to press lightly on the injection area for a few seconds.
- Do not rub the site.
Note: Store any unused syringes in the refrigerator in the original carton.

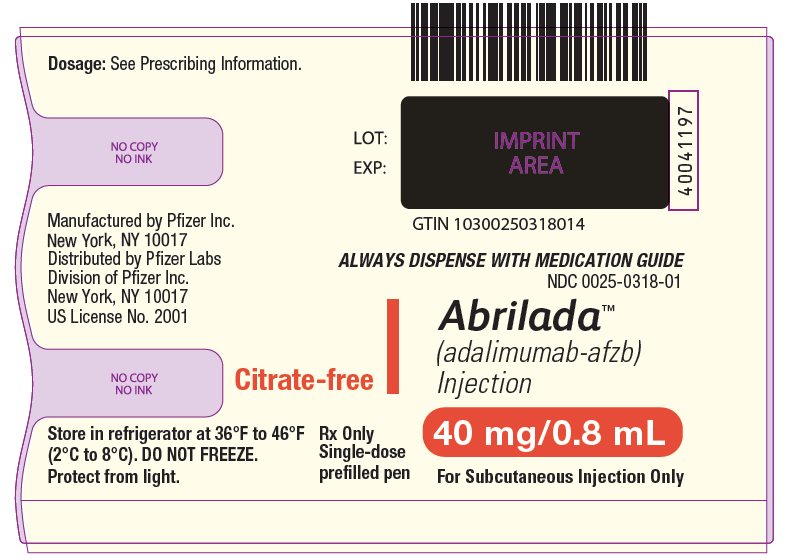
- 2 Prefilled Pens
- 2 Alcohol Preps
- 1 Prescribing Information
- 1 Instructions for Use
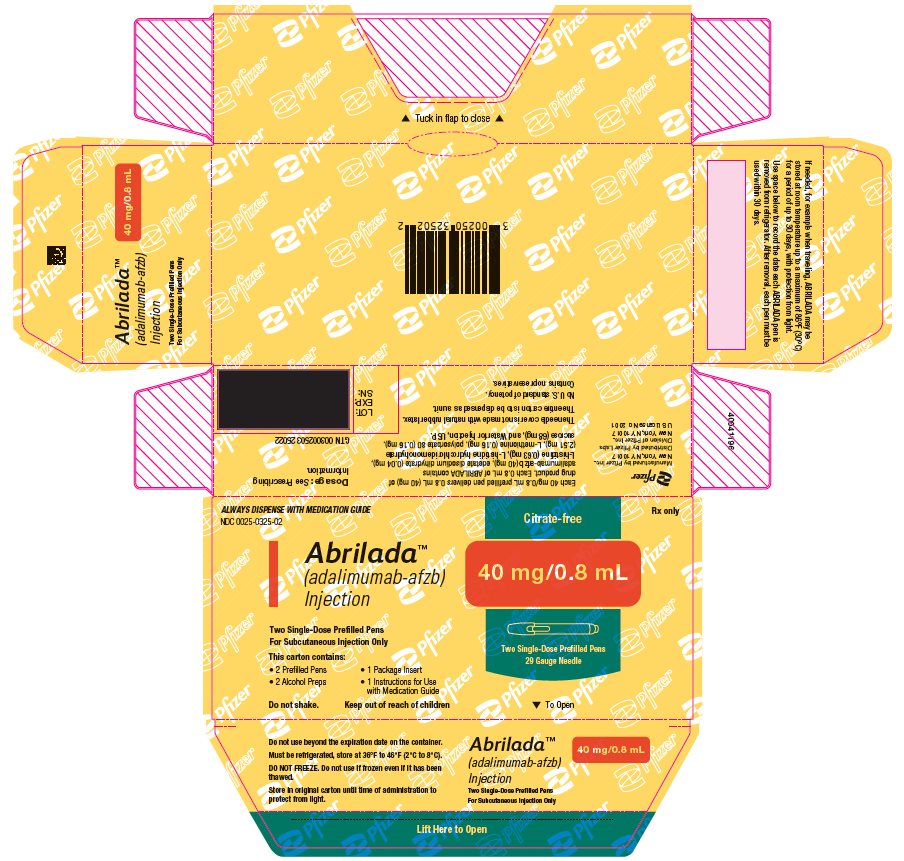

- 2 Prefilled Syringes
- 2 Alcohol Preps
- 1 Instructions for Use
- 1 Medication Guide
- 1 Prescribing Information
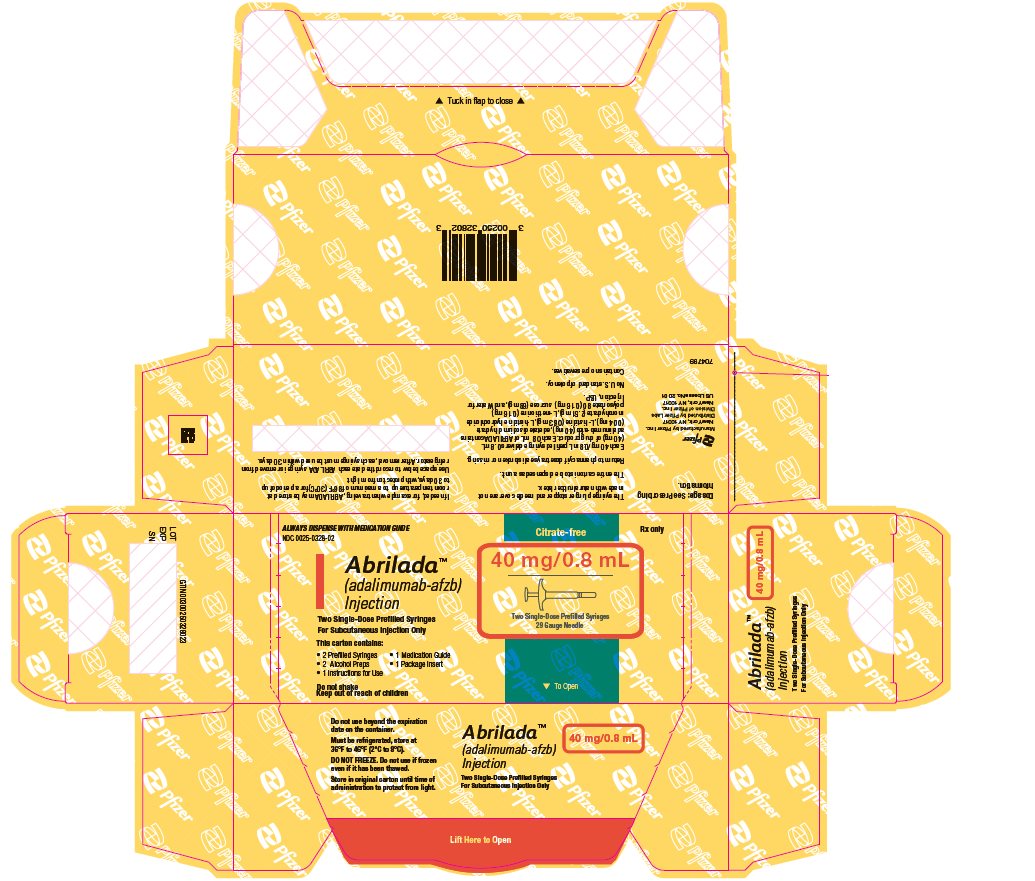


- 2 Prefilled Syringes
- 2 Alcohol Preps
- 1 Instructions for Use
- 1 Medication Guide
- 1 Prescribing Information
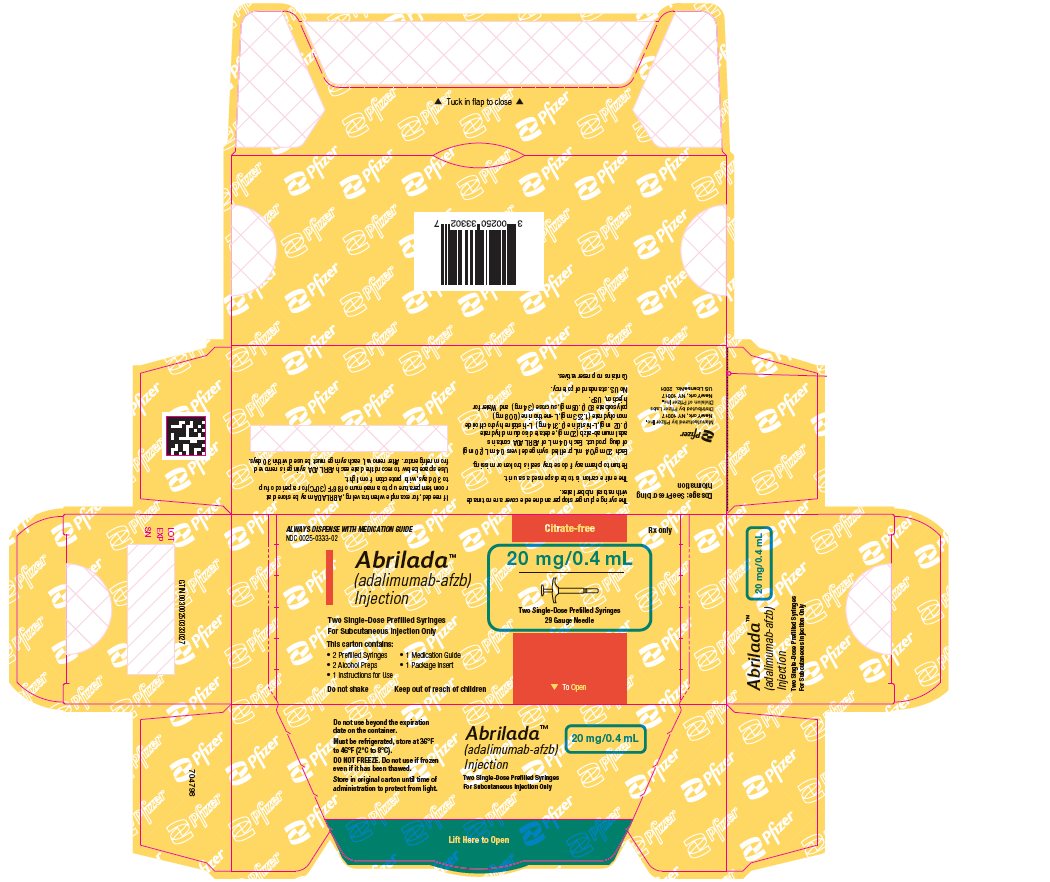

- 2 Prefilled Syringes
- 2 Alcohol Swabs
- 1 Instructions for Use
- 1 Medication Guide
- 1 Package Insert