Generic Name
SIrolimus
Brand Names
Fyarro, Hyftor, Rapamune
FDA approval date: July 01, 2001
Classification: mTOR Inhibitor Immunosuppressant
Form: Solution, Injection, Tablet, Gel
What is Fyarro (SIrolimus)?
FYARRO ™ is indicated for the treatment of adult patients with locally advanced unresectable or metastatic malignant perivascular epithelioid cell tumor . FYARRO is an mTOR inhibitor indicated for the treatment of adult patients with locally advanced unresectable or metastatic malignant perivascular epithelioid cell tumor .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
FYARRO (Sirolimus)
1INDICATIONS AND USAGE
FYARRO
2DOSAGE FORMS AND STRENGTHS
For injectable suspension: white to yellow, sterile lyophilized powder containing 100 mg of sirolimus formulated as albumin-bound particles in single-dose vial for reconstitution.
3CONTRAINDICATIONS
FYARRO is contraindicated in patients with a history of severe hypersensitivity to sirolimus, other rapamycin derivatives, or albumin
4ADVERSE REACTIONS
The following adverse reactions have been associated with FYARRO in clinical trials and are discussed in greater detail in other sections of the label
Stomatitis
Myelosuppression
Infections
Hypokalemia
Hyperglycemia
Interstitial Lung Disease (ILD) / Non-Infectious Pneumonitis
Hemorrhage
Hypersensitivity
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of FYARRO was assessed in a single-arm study (AMPECT). Thirty-four patients received FYARRO 100 mg/m
The median age of patients who received FYARRO was 59.5 years (range 27 to 78 years), 82% were female and Eastern Cooperative Oncology Group (ECOG) Performance Status was 0 (76%) or 1 (24%). Race was 71% White, 9% Black, 9% Asian, 3% Hawaiian/Pacific Islander and 9% Other/Not Reported. Ethnicity was 82% not Hispanic or Latino, 15% Hispanic or Latino, and 3% Not Reported.
Serious adverse reactions occurred in 14 (41%) patients who received FYARRO. Serious adverse reactions in >5% of patients, including 4 (12%) patients with infection and 2 (6%) patients each with abdominal pain, dehydration, and upper gastrointestinal hemorrhage. Fatal adverse reactions occurred in 1 (2.9%) patient who received FYARRO and experienced upper gastrointestinal hemorrhage.
Permanent discontinuation of FYARRO due to an adverse reaction occurred in 3 (9%) patients. Adverse reactions which resulted in permanent discontinuation of FYARRO included pneumonitis, anemia, and noninfective cystitis.
Dosage interruptions of FYARRO due to an adverse reaction occurred in 22 (65%) patients. Adverse reactions which required dosage interruption in >5% of patients included stomatitis in 6 (18%) patients, pneumonitis in 5 (15%) patients, anemia in 3 (9%) patients, and dehydration, dermatitis acneiform, and thrombocytopenia in 2 (6%) patients each.
Dose reductions of FYARRO due to an adverse reaction occurred in 12 (35%) patients. Adverse reactions which required dose reductions in >5% of patients included stomatitis and pneumonitis in 3 (9%) patients each.
The most common adverse reactions (≥30%) were stomatitis in 27 (79%) patients, fatigue and rash in 23 (68%) patients each, infection in 20 (59%) patients, nausea and edema in 17 (50%) patients each, diarrhea, musculoskeletal pain and decreased weight in 16 (47%) patients each, decreased appetite in 15 (44%) patients, cough in 12 (35%) patients, and vomiting and dysgeusia in 11 (32%) patients each. The most common Grade 3 to 4 laboratory abnormalities (≥6%) were decreased lymphocytes in 7 (21%) patients, increased glucose and decreased potassium in 4 (12%) patients each, decreased phosphate in 3 (9%) patients, and decreased hemoglobin and increased lipase in 2 (6%) patients each.
Table 4 summarizes the adverse reactions in AMPECT.
Table 5 summarizes the laboratory abnormalities in AMPECT.
Clinically relevant adverse reactions occurring in <10% of patients included enteritis, edema, pancytopenia, acute kidney injury, and acute coronary syndrome.
5DESCRIPTION
FYARRO (sirolimus protein-bound particles for injectable suspension) (albumin-bound) is sirolimus formulated as albumin-bound nanoparticles. The active ingredient in FYARRO is sirolimus bound to albumin which exists in the nanoparticles in a non-crystalline, amorphous state.
Sirolimus is a mechanistic target of rapamycin kinase (mTOR) inhibitor. Sirolimus is a macrocyclic lactone produced by
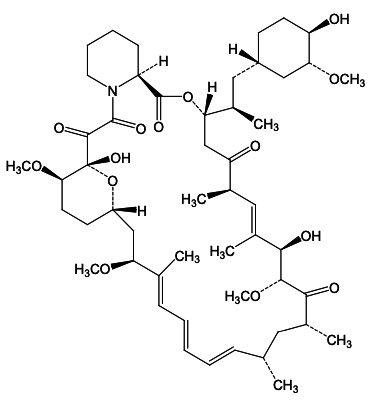
Sirolimus is a white to off-white crystalline powder and is insoluble in water but freely soluble in benzyl alcohol, chloroform, acetone, and acetonitrile.
FYARRO is supplied as a white to yellow, sterile, lyophilized powder for reconstitution with 20 mL of 0.9% Sodium Chloride Injection, USP prior to intravenous infusion. Each single-dose vial contains 100 mg of sirolimus (bound to human albumin) and approximately 850 mg of human albumin (containing sodium caprylate and sodium acetyltryptophanate). Each milliliter (mL) of reconstituted suspension contains 5 mg sirolimus formulated as albumin-bound particles.
6REFERENCES
1. OSHA Hazardous Drugs. OSHA. https://www.osha.gov/SLTC/hazardousdrugs/index.html.
7HOW SUPPLIED/STORAGE AND HANDLING
FYARRO (sirolimus protein-bound particles for injectable suspension) (albumin-bound) is a white to yellow, sterile lyophilized powder supplied as:
NDC 80803-153-50, 100 mg of sirolimus in a single-dose vial. Each carton contains 1 vial.
Store the vials in the original cartons at 2° to 8°C [USP Refrigerated Temperature] (36° to 46°F).
Retain in the original package to protect from light.
FYARRO is a hazardous drug. Follow applicable special handling and disposal procedures.
8PATIENT COUNSELING INFORMATION
Stomatitis
Advise patients of the risk of stomatitis
Myelosuppression
Advise patients of the risk of myelosuppression and the need to monitor blood counts periodically during therapy
Infections
Advise patients that they are more susceptible to infections and that they should immediately report any signs or symptoms of infection to their healthcare provider
Hypokalemia
Advise patients of the risk of hypokalemia and the need to monitor potassium periodically during therapy
Hyperglycemia
Advise patients of the risk of hyperglycemia and the need to monitor glucose periodically during therapy
Interstitial Lung Disease / Non-Infectious Pneumonitis
Advise patients of the risk of developing non-infectious pneumonitis and to immediately report any new or worsening respiratory symptoms to their healthcare provider
Hemorrhage
Advise patients of the risk of hemorrhage. Instruct patients to report signs of bleeding, and to seek immediate medical attention for signs or symptoms of severe bleeding
Hypersensitivity Reactions
Advise patients of the risk of clinically significant hypersensitivity reactions and to promptly contact their healthcare provider or seek emergency care for signs of hypersensitivity reaction including rash, itching, hives, difficulty breathing or swallowing, flushing, chest pain, or dizziness
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy
Advise males with female partners of reproductive potential to use effective contraception during treatment and for 12 weeks after the last dose
Infertility
Advise males and females of reproductive potential of the potential risk for impaired fertility
Immunizations
Advise patients that vaccinations may be less effective while being treated with FYARRO. Advise patients to avoid the use of live vaccines, and close contact with those who have received live vaccines, while on FYARRO
Lactation
Advise women not to breastfeed during treatment with FYARRO and for 2 weeks after the last dose
Drug Interactions
Advise patients to inform their healthcare provider about all concomitant medications, including prescription medicine, over the counter drugs, vitamins, and herbal products
This product's label may have been updated. For current full prescribing information, please visit www.aadibio.com.
Manufactured for Aadi Bioscience, Inc., Pacific Palisades, CA 90272.
FYARRO is a trademark of Aadi Bioscience, Inc.
Patent: www.aadibio.com/patents/
9PRINCIPAL DISPLAY PANEL
NDC 80803-153-50 Rx Only
