Generic Name
Amiodarone
Brand Names
Pacerone, Nexterone, Amiodarone HCI
FDA approval date: March 30, 2001
Classification: Antiarrhythmic
Form: Injection, Tablet
What is Pacerone (Amiodarone)?
Amiodarone hydrochloride is indicated for the treatment of documented, life-threatening recurrent ventricular fibrillation and life-threatening recurrent hemodynamically unstable tachycardia in adults who have not responded to adequate doses of other available antiarrhythmics or when alternative agents cannot be tolerated. Amiodarone hydrochloride is an antiarrhythmic indicated for: Recurrent ventricular fibrillation. Recurrent hemodynamically unstable ventricular tachycardia.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Pacerone (Amiodarone Hydrochloride)
WARNING: PULMONARY, HEPATIC and CARDIAC TOXICITY
Pacerone is intended for use only in patients with the indicated life-threatening arrhythmias because its use is accompanied by substantial toxicity
Pacerone can cause pulmonary toxicity (hypersensitivity pneumonitis or interstitial/alveolar pneumonitis) that has resulted in clinically manifest disease at rates as high as 17% in some series of patients. Pulmonary toxicity has been fatal about 10% of the time. Obtain a baseline chest X-ray and pulmonary-function tests, including diffusion capacity, when Pacerone therapy is initiated. Repeat history, physical exam, and chest X-ray every 3 to 6 months
Pacerone can cause hepatoxicity, which can be fatal. Obtain baseline and periodic liver transaminases and discontinue or reduce dose if the increase exceeds three times normal, or doubles in a patient with an elevated baseline. Discontinue Pacerone if the patient experiences signs or symptoms of clinical liver injury
Pacerone can exacerbate arrhythmias. Initiate amiodarone hydrochloride in a clinical setting where continuous electrocardiograms and cardiac resuscitation are available
1INDICATIONS AND USAGE
Pacerone is indicated for the treatment of documented, life-threatening recurrent ventricular fibrillation and life-threatening recurrent hemodynamically unstable tachycardia in adults who have not responded to adequate doses of other available antiarrhythmics or when alternative agents cannot be tolerated.
2DOSAGE AND ADMINISTRATION
Dosage must be individualized based on severity of arrhythmia and response. Use the lowest effective dose. Obtain baseline chest x-ray, pulmonary function tests, thyroid function tests, and liver aminotransferases. Correct hypokalemia, hypomagnesemia, and hypocalcemia before initiating treatment.
3DOSAGE FORMS AND STRENGTHS
Pacerone tablets, 100 mg, are peach, round, flat-faced, uncoated tablets, debossed with "P" on one side, and "U-S" above "144" on the other side.
Pacerone tablets, 200 mg, are pink, round, flat-faced, scored, uncoated tablets, debossed with "P
Pacerone tablets, 400 mg, are white to off-white, round, uncoated, scored tablets, debossed with "U-S' above and "1645' below the score on one side and "P
4CONTRAINDICATIONS
- Cardiogenic shock.
- Sick sinus syndrome, second- or third-degree atrioventricular block, bradycardia leading to syncope without a functioning pacemaker.
- Known hypersensitivity to the drug or to any of its components, including iodine.
5ADVERSE REACTIONS
The following serious adverse reactions are described in more detail in other sections of the prescribing information:
- Pulmonary Toxicity
- Hepatic Injury
- Worsened Arrhythmia
- Visual Impairment and Loss of Vision
- Thyroid Abnormalities
- Bradycardia
- Peripheral Neuropathy
- Photosensitivity and Skin Discoloration
5.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
At the usual maintenance dose (400 mg/day) and above, amiodarone hydrochloride causes adverse reactions in about three-fourths of all patients, resulting in discontinuation in 7% to 18%.
In surveys of almost 5,000 patients treated in open U.S. studies and in published reports of treatment with amiodarone hydrochloride, the adverse reactions most frequently requiring discontinuation of amiodarone hydrochloride included pulmonary infiltrates or fibrosis, paroxysmal ventricular tachycardia, congestive heart failure, and elevation of liver enzymes. Other symptoms causing discontinuations less often included visual disturbances, photosensitivity, blue skin discoloration, hyperthyroidism, and hypothyroidism.
The following side-effect rates are based on a retrospective study of 241 patients treated for 2 to 1,515 days (mean 441.3 days):
Thyroid
Common: Hypothyroidism, hyperthyroidism.
Cardiovascular
Common: Congestive heart failure, cardiac arrhythmias, SA node dysfunction.
Gastrointestinal
Very common: Nausea, vomiting.
Common: Constipation, anorexia, abdominal pain.
Dermatologic
Common: Solar dermatitis/photosensitivity.
Neurologic
Common: Malaise and fatigue, tremor/abnormal involuntary movements, lack of coordination, abnormal gait/ataxia, dizziness, paresthesias, decreased libido, insomnia, headache, sleep disturbances.
Ophthalmic
Common: Visual disturbances.
Hepatic
Common: Abnormal liver-function tests, nonspecific hepatic disorders.
Respiratory
Common: Pulmonary inflammation or fibrosis.
Other
Common: Flushing, abnormal taste and smell, edema, abnormal salivation, coagulation abnormalities.
Uncommon: Blue skin discoloration, rash, spontaneous ecchymosis, alopecia, hypotension, and cardiac conduction abnormalities.
5.2Post-marketing Experience
The following adverse reactions have been identified during post-approval use of amiodarone hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hematologic: hemolytic anemia, aplastic anemia, pancytopenia, neutropenia, thrombocytopenia, agranulocytosis, granuloma.
Immune: anaphylactic/anaphylactoid reaction (including shock), angioedema.
Neurologic: pseudotumor cerebri, parkinsonian symptoms such as akinesia and bradykinesia (sometimes reversible with discontinuation of therapy), demyelinating polyneuropathy.
Psychiatric: hallucination, confusional state, disorientation, delirium.
Cardiac: hypotension (sometimes fatal), sinus arrest.
Respiratory: eosinophilic pneumonia, acute respiratory distress syndrome in the post-operative setting, bronchospasm, bronchiolitis obliterans organizing pneumonia, pulmonary alveolar hemorrhage, pleural effusion, pleuritis.
Gastrointestinal: pancreatitis, acute pancreatitis.
Hepatic: hepatitis, cholestatic hepatitis, cirrhosis.
Skin and Subcutaneous Tissue Disorders: urticaria, toxic epidermal necrolysis (sometimes fatal), erythema multiforme, Stevens-Johnson syndrome, exfoliative dermatitis, bullous dermatitis, drug rash with eosinophilia and systemic symptoms (DRESS), eczema, pruritus, skin cancer, lupus-like syndrome.
Musculoskeletal: myopathy, muscle weakness, rhabdomyolysis.
Renal: renal impairment, renal insufficiency, acute renal failure.
Reproductive: epididymitis, impotence.
Body as a whole: fever, dry mouth.
Endocrine and metabolic: thyroid nodules/ thyroid cancer, syndrome of inappropriate antidiuretic hormone secretion (SIADH).
Vascular: vasculitis.
6DRUG INTERACTIONS
Because of amiodarone's long half-life, expect drug interactions to persist for weeks to months after discontinuation of amiodarone.
Drug interactions with amiodarone are described in Table 1 below.
7OVERDOSAGE
There have been cases, some fatal, of amiodarone hydrochloride overdose.
Monitor the patient's cardiac rhythm and blood pressure, and, if bradycardia ensues, a β-adrenergic agonist or a pacemaker may be used. Treat hypotension with inadequate tissue perfusion with positive inotropic and vasopressor agents. Neither Pacerone nor its metabolite is dialyzable.
8DESCRIPTION
Pacerone (amiodarone hydrochloride tablets, USP) are an antiarrhythmic drug, available for oral administration as 100 mg; peach tablets, 200 mg; pink tablets and 400 mg; white tablets containing amiodarone hydrochloride, USP. All three strengths of Pacerone tablets contain the following inactive ingredients: lactose monohydrate, magnesium stearate, povidone, pregelatinized corn starch, sodium starch glycolate and stearic acid. The 200 mg tablets also contain FD&C Red No. 40 and FD&C Yellow No. 6. The 100 mg tablets also contain FD&C Yellow No. 6.
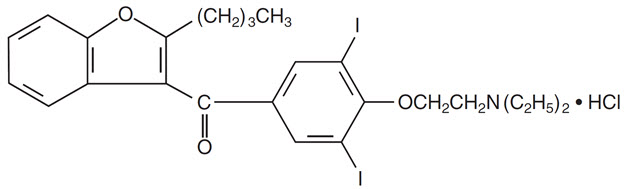
Amiodarone hydrochloride, USP is a benzofuran derivative: 2-butyl-3-benzofuranyl 4-[2-(diethylamino)-ethoxy]-3,5-diiodophenyl ketone hydrochloride.
The structural formula is as follows:
C
C
Amiodarone hydrochloride, USP is a white to cream-colored crystalline powder. It is slightly soluble in water, soluble in alcohol, and freely soluble in chloroform. It contains 37.3% iodine by weight.
Meets USP Dissolution Test 4.
9HOW SUPPLIED/STORAGE AND HANDLING
Pacerone
Bottles of 30 with child-resistant closure, NDC 0245-0144-30
Bottles of 100 with child-resistant closure, NDC 0245-0144-11
Unit-dose cartons of 100 tablets, NDC 0245-0144-01
Pacerone
Bottles of 60 with child-resistant closure, NDC 0245-0147-60
Bottles of 90 with child-resistant closure, NDC 0245-0147-90
Bottles of 500 tablets, NDC 0245-0147-15
Unit-dose cartons of 100 tablets, NDC 0245-0147-01
Pacerone
Bottles of 30 with child-resistant closure, NDC 0245-1645-30
Unit-dose cartons of 100 tablets, NDC 0245-1645-01
Keep tightly closed.
10PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to inform their prescriber of a known or suspected pregnancy
Advise women that breastfeeding is not recommended during treatment with Pacerone
Advise patients to avoid grapefruit juice and St. John's Wort.
Advise patients to seek medical attention if they experience the signs and symptoms of pulmonary toxicity, worsening arrhythmia, bradycardia, visual impairment, or hypo- and hyperthyroidism.
This product's label may have been updated. For full prescribing information, please visit www.upsher-smith.com.
11Repackaging Information
Please reference the
Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:
12PRINCIPAL DISPLAY PANEL - 200 mg
NDC 71610-787 - Amiodarone HCl (Pacerone) 200 mg Tablets - Rx Only