Brand Name
Benicar
Generic Name
Olmesartan medoxomil-
View Brand Information FDA approval date: September 01, 2022
Classification: Thiazide Diuretic
Form: Tablet
What is Benicar (Olmesartan medoxomil-)?
BENICAR HCT is indicated for the treatment of hypertension, to lower blood pressure. BENICAR HCT is not indicated for the initial therapy of hypertension. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with BENICAR HCT. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure . Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension , and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects in black patients, and many antihypertensive drugs have additional approved indications and effects . These considerations may guide selection of therapy. BENICAR HCT may be used alone, or in combination with other antihypertensive drugs. BENICAR HCT is a combination of olmesartan, an angiotensin II receptor blocker and hydrochlorothiazide, a thiazide diuretic indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Benicar HCT (olmesartan medoxomil-hydrochlorothiazide)
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue BENICAR HCT as soon as possible[seeWarnings and Precautions (5.1)].
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus[seeWarnings and Precautions (5.1)].
1INDICATIONS AND USAGE
BENICAR HCT (olmesartan medoxomil and hydrochlorothiazide) is indicated for the treatment of hypertension, to lower blood pressure. BENICAR HCT is not indicated for the initial therapy of hypertension
Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with BENICAR HCT.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
BENICAR HCT may be used alone, or in combination with other antihypertensive drugs.
2DOSAGE AND ADMINISTRATION
The recommended starting dose of BENICAR HCT is 40/12.5 mg once daily in patients whose blood pressure is not adequately controlled with olmesartan monotherapy. Dose can be titrated up to 40 /25 mg if necessary.
The recommended starting dose of BENICAR HCT is 20/12.5 mg once daily in patients whose blood pressure is not adequately controlled with HCT monotherapy or who experience dose-limiting adverse reactions with hydrochlorothiazide. Dose can be titrated up to 40 /25 mg if necessary.
Patients titrated to the individual components (olmesartan and hydrochlorothiazide) may instead receive the corresponding dose of BENICAR HCT.
3DOSAGE FORMS AND STRENGTHS
BENICAR HCT (olmesartan / hydrochlorothiazide) is supplied as film-coated, non-scored tablets:
- 20 mg/12.5 mg reddish-yellow, circular, debossed with Sankyo on one side and C22 on the other side
- 40 mg/12.5 mg reddish-yellow, oval, debossed with Sankyo on one side and C23 on the other side
- 40 mg/25 mg pink, oval, debossed with Sankyo on one side and C25 on the other side
4CONTRAINDICATIONS
BENICAR HCT is contraindicated:
- In patients with hypersensitivity to any component of BENICAR HCT
- In patients with anuria
- For coadministration with aliskiren in patients with diabetes
5ADVERSE REACTIONS
The following adverse reactions with BENICAR HCT are described elsewhere:
- Hypotension in Volume- or Salt-Depleted Patients
- Impaired Renal Function
- Hypersensitivity Reactions
- Electrolyte and Metabolic Imbalances
- Acute Myopia and Secondary Angle-Closure Glaucoma
- Systemic Lupus Erythematosus
- Sprue-Like Enteropathy
5.1Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Olmesartan medoxomilandhydrochlorothiazide
The concomitant use of olmesartan medoxomil and hydrochlorothiazide was evaluated for safety in 1243 hypertensive patients. Treatment with olmesartan medoxomil and hydrochlorothiazide was well tolerated, with an incidence of adverse events similar to that of placebo. Adverse reactions were generally mild, transient and not dependent on the dose of olmesartan medoxomil and hydrochlorothiazide.
The rate of withdrawals for adverse events in all trials of hypertensive patients was 2.0% (25/1243) on olmesartan medoxomil plus hydrochlorothiazide and 2.0% (7/342) on placebo.
In a placebo-controlled, factorial clinical trial of olmesartan medoxomil (2.5 mg to 40 mg) and hydrochlorothiazide (12.5 mg to 25 mg), the following adverse reactions reported in Table 1 occurred in >2% of patients, and more often on the olmesartan medoxomil and hydrochlorothiazide combination than on placebo.
Other adverse reactions that have been reported with an incidence of greater than 1.0%, whether or not attributed to treatment, in the more than 1200 hypertensive patients treated with olmesartan medoxomil and hydrochlorothiazide in controlled or open-label trials are listed below.
Body as a Whole:chest pain, back pain, peripheral edema
Central and Peripheral Nervous System:vertigo
Gastrointestinal:abdominal pain, dyspepsia, gastroenteritis, diarrhea
Liver and Biliary System:SGOT increased, GGT increased, ALT increased
Metabolic and Nutritional:creatine phosphokinase increased
Musculoskeletal:arthritis, arthralgia, myalgia
Respiratory System:coughing
Skin and Appendages Disorders:rash
Urinary System:hematuria
Central and Peripheral Nervous System:vertigo
Gastrointestinal:abdominal pain, dyspepsia, gastroenteritis, diarrhea
Liver and Biliary System:SGOT increased, GGT increased, ALT increased
Metabolic and Nutritional:creatine phosphokinase increased
Musculoskeletal:arthritis, arthralgia, myalgia
Respiratory System:coughing
Skin and Appendages Disorders:rash
Urinary System:hematuria
Facial edema was reported in 2/1243 patients receiving olmesartan medoxomil and hydrochlorothiazide. Angioedema has been reported with angiotensin II receptor antagonists, including BENICAR HCT.
Hydrochlorothiazide
Other adverse reactions that have been reported with hydrochlorothiazide are listed below:
Body as a Whole:weakness
Digestive:pancreatitis, jaundice (intrahepatic cholestatic jaundice), sialadenitis, cramping, gastric irritation
Hematologic:aplastic anemia, agranulocytosis, leukopenia, hemolytic anemia, thrombocytopenia
Hypersensitivity:purpura, photosensitivity, urticaria, necrotizing angiitis (vasculitis and cutaneous vasculitis), fever, respiratory distress including pneumonitis and pulmonary edema, anaphylactic reactions
Metabolic:glycosuria, hyperuricemia
Musculoskeletal:muscle spasm
Nervous System/Psychiatric:restlessness
Renal:renal dysfunction, interstitial nephritis
Skin:erythema multiforme including Stevens-Johnson syndrome, exfoliative dermatitis including toxic epidermal necrolysis
Special Senses:transient blurred vision, xanthopsia
Digestive:pancreatitis, jaundice (intrahepatic cholestatic jaundice), sialadenitis, cramping, gastric irritation
Hematologic:aplastic anemia, agranulocytosis, leukopenia, hemolytic anemia, thrombocytopenia
Hypersensitivity:purpura, photosensitivity, urticaria, necrotizing angiitis (vasculitis and cutaneous vasculitis), fever, respiratory distress including pneumonitis and pulmonary edema, anaphylactic reactions
Metabolic:glycosuria, hyperuricemia
Musculoskeletal:muscle spasm
Nervous System/Psychiatric:restlessness
Renal:renal dysfunction, interstitial nephritis
Skin:erythema multiforme including Stevens-Johnson syndrome, exfoliative dermatitis including toxic epidermal necrolysis
Special Senses:transient blurred vision, xanthopsia
Clinical Laboratory Test Findings
Creatinine/bloodureanitrogen (BUN):Minor elevations in creatinine and BUN occurred in 1.7% and 2.5% respectively, of patients taking BENICAR HCT and 0% and 0% respectively, given placebo in controlled clinical trials.
5.2Post-marketing Experience
The following adverse reactions have been identified during post-approval use of BENICAR HCT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Body as a Whole:Asthenia
Gastrointestinal:Vomiting
Metabolic:Hyperkalemia
Musculoskeletal:Rhabdomyolysis
Skin and Appendages:Alopecia, pruritus
Gastrointestinal:Vomiting
Metabolic:Hyperkalemia
Musculoskeletal:Rhabdomyolysis
Skin and Appendages:Alopecia, pruritus
Data from one controlled trial and an epidemiologic study have suggested that high-dose olmesartan may increase cardiovascular (CV) risk in diabetic patients, but the overall data are not conclusive. The randomized, placebo-controlled, double-blind ROADMAP trial (Randomized Olmesartan And Diabetes MicroAlbuminuria Prevention trial, n=4447) examined the use of olmesartan, 40 mg daily, vs. placebo in patients with type 2 diabetes mellitus, normoalbuminuria, and at least one additional risk factor for CV disease.
The trial met its primary endpoint, delayed onset of microalbuminuria, but olmesartan had no beneficial effect on decline in glomerular filtration rate (GFR). There was a finding of increased CV mortality (adjudicated sudden cardiac death, fatal myocardial infarction, fatal stroke, revascularization death) in the olmesartan group compared to the placebo group (15 olmesartan vs. 3 placebo, HR 4.9, 95% confidence interval [CI], 1.4, 17), but the risk of non-fatal myocardial infarction was lower with olmesartan (HR 0.64, 95% CI 0.35, 1.18).
The epidemiologic study included patients 65 years and older with overall exposure of > 300,000 patient-years. In the sub-group of diabetic patients receiving high-dose olmesartan (40 mg/d) for > 6 months, there appeared to be an increased risk of death (HR 2.0, 95% CI 1.1, 3.8) compared to similar patients taking other angiotensin receptor blockers. In contrast, high-dose olmesartan use in non-diabetic patients appeared to be associated with a decreased risk of death (HR 0.46, 95% CI 0.24, 0.86) compared to similar patients taking other angiotensin receptor blockers. No differences were observed between the groups receiving lower doses of olmesartan compared to other angiotensin blockers or those receiving therapy for < 6 months.
Overall, these data raise a concern of a possible increased CV risk associated with the use of high-dose olmesartan in diabetic patients. There are, however, concerns with the credibility of the finding of increased CV risk, notably the observation in the large epidemiologic study for a survival benefit in non-diabetics of a magnitude similar to the adverse finding in diabetics.
6OVERDOSAGE
Olmesartan medoxomil
Limited data are available related to overdosage of olmesartan medoxomil in humans. The most likely manifestations of overdosage would be hypotension and tachycardia; bradycardia could be encountered if parasympathetic (vagal) stimulation occurs. If symptomatic hypotension should occur, supportive treatment should be initiated. The dialyzability of olmesartan is unknown.
No lethality was observed in acute toxicity studies in mice and rats given single oral doses up to 2000 mg/kg olmesartan medoxomil. The minimum lethal oral dose of olmesartan medoxomil in dogs was greater than 1500 mg/kg.
Hydrochlorothiazide
The most common signs and symptoms of hydrochlorothiazide overdose observed in humans are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias. The degree to which hydrochlorothiazide is removed by hemodialysis has not been established. The oral LD
7DESCRIPTION
BENICAR HCT (olmesartan medoxomil and hydrochlorothiazide) is a combination of an angiotensin II receptor antagonist (AT
Olmesartan medoxomil is 2,3-dihydroxy-2-butenyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[
Its empirical formula is C
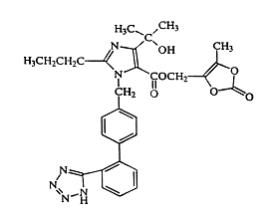
Olmesartan medoxomil is a white to light yellowish-white powder or crystalline powder with a molecular weight of 558.6. It is practically insoluble in water and sparingly soluble in methanol.
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2
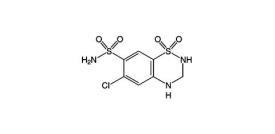
Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.7. Hydrochlorothiazide is slightly soluble in water but freely soluble in sodium hydroxide solution.
BENICAR HCT is available for oral administration in tablets containing 20 mg or 40 mg of olmesartan medoxomil combined with 12.5 mg of hydrochlorothiazide, or 40 mg of olmesartan medoxomil combined with 25 mg of hydrochlorothiazide. Inactive ingredients include: hydroxypropylcellulose, hypromellose, lactose monohydrate, low-substituted hydroxypropylcellulose, magnesium stearate, microcrystalline cellulose, red iron oxide, talc, titanium dioxide and yellow iron oxide.
8CLINICAL STUDIES
Olmesartan medoxomilandhydrochlorothiazide
In clinical trials 1230 patients were exposed to the combination of olmesartan medoxomil (2.5 mg to 40 mg) and hydrochlorothiazide (12.5 mg to 25 mg). These trials included one placebo-controlled factorial trial in mild-moderate hypertensive patients (n=502) with combinations of olmesartan medoxomil (10 mg, 20 mg, 40 mg, or placebo) and hydrochlorothiazide (12.5 mg, 25 mg, or placebo). The antihypertensive effect of the combination on trough blood pressure was related to the dose of each component (see Table 2).
Once-daily dosing with 20 mg olmesartan medoxomil and 12.5 mg hydrochlorothiazide, 40 mg olmesartan medoxomil and 12.5 mg hydrochlorothiazide or 40 mg olmesartan medoxomil and 25 mg hydrochlorothiazide produced mean placebo-adjusted blood pressure reductions at trough (24 hours post-dosing) ranging from 17/8 to 24/14 mm Hg.
The antihypertensive effect had onset within 1 week and was near maximal at 4 weeks. The antihypertensive effect was independent of gender, but there were too few subjects to identify response differences based on race or age greater than or less than 65 years. No appreciable changes in trough heart rate were observed with combination therapy.
There are no trials of BENICAR HCT demonstrating reductions in cardiovascular risk in patients with hypertension, but at least one drug pharmacologically similar to olmesartan medoxomil has demonstrated such benefits, and hydrochlorothiazide demonstrated reduction of cardiovascular risk in patients with hypertension.
Olmesartan medoxomil
The antihypertensive effects of olmesartan medoxomil have been demonstrated in seven placebo-controlled studies at doses ranging from 2.5 to 80 mg for 6 to 12 weeks, each showing statistically significant reductions in peak and trough blood pressure. A total of 2693 patients (2145 olmesartan medoxomil; 548 placebo) with essential hypertension were studied. Olmesartan medoxomil once daily (QD) lowered diastolic and systolic blood pressure. The response was dose-related. An olmesartan medoxomil dose of 20 mg daily produced a trough sitting BP reduction over placebo of about 10/6 mm Hg and a dose of 40 mg daily produced a trough sitting BP reduction over placebo of about 12/7 mm Hg. Olmesartan medoxomil doses greater than 40 mg had little additional effect. The onset of the antihypertensive effect occurred within 1 week and was largely manifest after 2 weeks.
The blood pressure lowering effect was maintained throughout the 24-hour period with olmesartan medoxomil once daily, with trough-to-peak ratios for systolic and diastolic response between 60 and 80%.
The blood pressure lowering effect of olmesartan medoxomil, with and without hydrochlorothiazide, was maintained in patients treated for up to 1 year. There was no evidence of tachyphylaxis during long-term treatment with olmesartan medoxomil or rebound effect following abrupt withdrawal of olmesartan medoxomil after 1 year of treatment.
The antihypertensive effect of olmesartan medoxomil was similar in men and women and in patients older and younger than 65 years. The effect was smaller in black patients (usually a low-renin population), as has been seen with other ACE inhibitors, angiotensin receptor blockers, and beta-blockers. Olmesartan medoxomil had an additional blood pressure lowering effect when added to hydrochlorothiazide.
9HOW SUPPLIED/STORAGE AND HANDLING
BENICAR HCT is supplied as follows:
Tablets are packaged as follows:
Storage
Store at 20-25ºC (68-77ºF) [See USP Controlled Room Temperature].
10PATIENT COUNSELING INFORMATION
Pregnancy:Advisefemale patients of childbearing age about the consequences of exposure to BENICAR HCT during pregnancy. Discuss treatment options with women planning to become pregnant. Tell patients to report pregnancies to their physicians as soon as possible [seeUse in Specific Populations (8.1)].
Symptomatichypotensionandsyncope:Advise patients that lightheadedness can occur, especially during the first days of therapy, and to report this symptom to a healthcare provider. Inform patients that dehydration from inadequate fluid intake, excessive perspiration, vomiting, or diarrhea may lead to an excessive fall in blood pressure. If syncope occurs advise patients, to contact their healthcare provider.
Potassium Supplements: Advise patients not to use potassium supplements or salt substitutes containing potassium without consulting their healthcare provider.
Acutemyopia andsecondaryangle-closureglaucoma:Advise patients to discontinue BENICAR HCT and seek immediate medical attention if they experience symptoms of acute myopia or secondary angle-closure glaucoma [seeWarnings and Precautions (5.6)].
Non-melanoma Skin Cancer:Instruct patients taking hydrochlorothiazide to protect skin from the sun and undergo regular skin cancer screening.
Marketed by Cosette Pharmaceuticals, Inc. South Plainfield, NJ 07080
8-BENHCP1 Iss. 02/2022
11PRINCIPAL DISPLAY PANEL
NDC 0713-0863-30

12PRINCIPAL DISPLAY PANEL
NDC 0713-0864-30

13PRINCIPAL DISPLAY PANEL
NDC 0713-0865-30
