Brand Name
Velsipity
Generic Name
Etrasimod
View Brand Information FDA approval date: October 19, 2023
Classification: Sphingosine 1-phosphate Receptor Modulator
Form: Tablet
What is Velsipity (Etrasimod)?
VELSIPITY is indicated for the treatment of moderately to severely active ulcerative colitis in adults. VELSIPITY is a sphingosine 1-phosphate receptor modulator indicated for the treatment of moderately to severely active ulcerative colitis in adults.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Velsipity (etrasimod)
1INDICATIONS AND USAGE
VELSIPITY is indicated for the treatment of moderately to severely active ulcerative colitis (UC) in adults.
2DOSAGE FORMS AND STRENGTHS
Tablets: 2 mg of etrasimod as a round, green, film-coated tablet, debossed with “ETR” on one side and “2” on the other side.
3CONTRAINDICATIONS
VELSIPITY is contraindicated in patients who:
- In the last 6 months, have experienced a myocardial infarction, unstable angina pectoris, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III or IV heart failure
- Have a history or presence of Mobitz type II second-degree or third-degree AV block, sick sinus syndrome, or sino-atrial block, unless the patient has a functioning pacemaker
4ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Infections
- Bradyarrhythmia and Atrioventricular Conduction Delays
- Liver Injury
- Macular Edema
- Increased Blood Pressure
- Fetal Risk
- Cutaneous Malignancies
- Posterior Reversible Encephalopathy Syndrome
- Respiratory Effects
- Unintended Additive Immune System Effects from Prior Treatment with Immunosuppressive or Immune-Modulating Drugs
- Immune System Effects After Stopping VELSIPITY
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of VELSIPITY 2 mg once daily in subjects with moderately to severely active ulcerative colitis was evaluated in two randomized, placebo-controlled studies of 52 weeks (UC-1) and 12 weeks (UC-2) duration
In the 52-week study (UC-1), 433 subjects were enrolled of whom 289 received VELSIPITY 2 mg once daily. In the 12-week studies (UC-2 and UC-3), 458 subjects were enrolled of whom 288 received VELSIPITY 2 mg once daily.
Table 1 summarizes the adverse reactions reported in at least 2% of subjects and at a higher rate than placebo during UC-1.
Table 2 summarizes the adverse reactions reported in at least 2% of subjects and at a higher rate than placebo during UC-2 and UC-3.
Ophthalmologic Findings
In UC-1, for subjects with a baseline and follow-up examination, a decrease in visual acuity was reported in 2.6% (4/156) of subjects who received VELSIPITY and no subjects who received placebo
5DRUG INTERACTIONS
Etrasimod is primarily metabolized by CYP2C8, CYP2C9, and CYP3A4. Table 3 includes drugs with clinically important drug interactions when administered concomitantly with VELSIPITY and instructions for preventing or managing them. Consult the labeling of concomitantly used drugs to obtain further information.
The effect of concomitant use of VELSIPITY with a combination of separate drugs that are moderate to strong inhibitors or inducers of either CYP2C8, CYP2C9, or CYP3A4 is unknown. However, based on the information below, a similar clinically significant change in exposure cannot be ruled out when two or more metabolic pathways are affected.
6DESCRIPTION
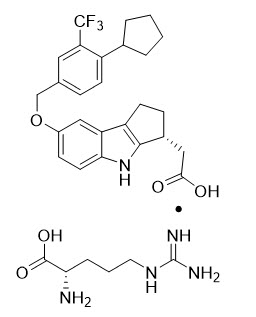
VELSIPITY contains etrasimod, a sphingosine 1-phosphate (S1P) receptor modulator, supplied as etrasimod arginine.
Etrasimod arginine is a white, off-white to light brown solid that is slightly soluble in water. The chemical name of etrasimod arginine is L-Arginine, (3
The chemical structure of etrasimod arginine is:
VELSIPITY is supplied for oral administration as 2 mg tablets. Each tablet contains 2 mg etrasimod (equivalent to 2.76 mg etrasimod arginine) and the following inactive ingredients: magnesium stearate, mannitol, microcrystalline cellulose, and sodium starch glycolate, with a film coating containing FD&C blue #1/brilliant blue FCF aluminum lake, FD&C blue #2/indigo carmine aluminum lake, FD&C yellow #5/tartrazine aluminum lake, macrogol 4000 JP/PEG 3350, polyvinyl alcohol (partially hydrolyzed), talc, and titanium dioxide.
7CLINICAL STUDIES
The efficacy of VELSIPITY was evaluated in 2 randomized, double-blind, placebo-controlled clinical studies [UC-1 (NCT03945188) and UC-2 (NCT03996369)] in adult subjects with moderately to severely active ulcerative colitis who had an inadequate response, loss of response, or intolerance to one or more of the following treatment options: oral aminosalicylates, corticosteroids, thiopurines, Janus kinase (JAK) inhibitors, or biologic therapies (e.g., TNF blocker, anti-integrin, anti-IL12/23). UC-1 was a 52-week study and UC-2 was a 12-week study. In both studies, subjects were randomized to VELSIPITY or placebo and continued on treatment for the entire duration of the study.
Disease severity was assessed based on the modified Mayo score (mMS), a 3-component Mayo score (0 to 9) which consists of the following subscores (0 to 3 for each subscore): stool frequency (SF), rectal bleeding (RB), and findings on centrally read endoscopy score (ES). An ES of 2 was defined by marked erythema, lack of vascular pattern, any friability, and/or erosions, and a score of 3 was defined by spontaneous bleeding and ulceration.
Subjects in these studies may have received other concomitant UC therapies including stable daily doses of oral aminosalicylates and/or oral corticosteroids (≤20 mg/day prednisone, ≤9 mg/day budesonide, or equivalent steroid). Concomitant treatment with immunomodulators (e.g., thiopurines, methotrexate), biologic therapies, JAK inhibitors, rectal 5-ASA, or rectal corticosteroids was not permitted.
Endpoints and Results Study UC-1
In UC-1, efficacy was evaluated in 408 adult subjects with a baseline mMS of 5 to 9 (median of 7), including a centrally read endoscopy subscore of 2 or 3. Subjects were randomized 2:1 to receive VELSIPITY 2 mg or placebo administered orally once daily. Subjects had a mean age of 41 years (range 18 to 78 years); 45% were female; and 89% identified as White, 7% as Asian, 2% as Black or African American, 1% as American Indian or Alaska Native, and 1% did not report their racial group. A total of 30% of subjects had prior exposure to biologic/JAK inhibitors and a total of 14% of subjects had exposure to >1 biologic/JAK inhibitor. At baseline, 68% of subjects were receiving oral aminosalicylates and 31% of subjects were receiving oral corticosteroids.
The coprimary endpoints were the proportion of subjects achieving clinical remission at Week 12 and at Week 52. The secondary endpoints included the proportion of subjects achieving endoscopic improvement, histologic-endoscopic mucosal improvement, corticosteroid-free clinical remission, and maintenance of clinical remission (see Table 4).
The relationship of histologic-endoscopic mucosal improvement at Week 12 or Week 52 to disease progression and longer-term outcomes after Week 52 was not evaluated in Study UC-1.
Clinical Response
A greater proportion of subjects treated with VELSIPITY compared to placebo achieved clinical response, defined as a ≥2 point and ≥30% decrease from baseline in mMS, and a ≥1 point decrease from baseline in RB subscore or an absolute RB subscore ≤1 at Week 12 (62% vs 34%).
Stool Frequency and Rectal Bleeding Subscores
Decreases in SF subscores were observed as early as Week 2 and decreases in RB subscores were observed as early as Week 4 in subjects treated with VELSIPITY compared to placebo.
Endoscopic Assessment
Normalization of the endoscopic appearance of the mucosa (endoscopic remission) was defined as ES of 0. A greater proportion of subjects treated with VELSIPITY compared to placebo achieved endoscopic remission by Week 12 (15% vs 4%), Week 52 (26% vs 6%), and both Week 12 and Week 52 (11% vs 1%).
Endpoints and Results Study UC-2
In UC-2, efficacy was evaluated in 333 adult subjects with a baseline mMS of 5 to 9 (median of 7), including a centrally read endoscopy subscore of 2 or 3. Subjects were randomized 2:1 to receive VELSIPITY 2 mg or placebo administered orally once daily. Subjects had a mean age of 41 years (range 18 to 73 years); 41% were female; and 76% identified as White, 19% as Asian, 2% as American Indian or Alaska Native, 1% as Black or African American, and 2% as multiple racial groups or did not report their racial group. A total of 34% of subjects had prior exposure to biologic/JAK inhibitors and a total of 17% of subjects had exposure to >1 biologic/JAK inhibitor. At baseline, 66% of subjects were receiving oral aminosalicylates and 28% of subjects were receiving oral corticosteroids.
The primary endpoint was the proportion of subjects achieving clinical remission at Week 12. The secondary endpoints included the proportion of subjects achieving endoscopic improvement and histologic-endoscopic mucosal improvement at Week 12 (see Table 5).
The relationship of histologic-endoscopic mucosal improvement at Week 12 to disease progression and longer-term outcomes after Week 12 was not evaluated in Study UC-2.
Clinical Response
A greater proportion of subjects treated with VELSIPITY compared to placebo achieved clinical response, defined as a ≥2 point and ≥30% decrease from baseline in mMS, and a ≥1 point decrease from baseline in RB subscore or an absolute RB subscore ≤1 at Week 12 (62% vs 41%).
Stool Frequency and Rectal Bleeding Subscores
Decreases in SF subscores were observed as early as Week 2 and decreases in RB subscores were observed as early as Week 4 in subjects treated with VELSIPITY compared to placebo.
Endoscopic Assessment
Normalization of the endoscopic appearance of the mucosa (endoscopic remission) was defined as ES of 0. A greater proportion of subjects treated with VELSIPITY compared to placebo achieved endoscopic remission by Week 12 (17% vs 8%).
8HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
VELSIPITY tablet is available as a round, green film-coated 2 mg etrasimod tablet, debossed with “ETR” on one side and “2” on the other side.
VELSIPITY tablets are packed in a child-resistant 100 mL white, round, high-density polyethylene (HDPE) bottle containing 4 g of silica gel desiccant integrated directly into the 45 mm polypropylene (PP) cap.
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Risk of Infections
Inform patients that they may be more likely to get infections, some of which could be life-threatening, when taking VELSIPITY and for 5 weeks after stopping it, and that they should contact their healthcare provider if they develop symptoms of infection
Advise patients that some vaccines containing live virus (live
Cardiac Effects
Advise patients that initiation of VELSIPITY treatment may result in transient decrease in heart rate
Liver Injury
Inform patients that VELSIPITY may increase liver enzymes. Advise patients that they should contact their healthcare provider if they have any unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine
Macular Edema
Advise patients that VELSIPITY may cause macular edema, and that they should obtain an eye exam near the start of treatment with VELSIPITY, have their eyes monitored periodically by an eye care professional while receiving therapy, and contact their healthcare provider if they experience any changes in their vision while taking VELSIPITY
Fetal Risk
VELSIPITY may cause fetal harm. Advise females to immediately inform their healthcare provider of a known or suspected pregnancy
Advise females of reproductive potential to use effective contraception during treatment with VELSIPITY and for one week after stopping VELSIPITY
Pregnancy and Pregnancy Registry
Advise patients that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to VELSIPITY during pregnancy
Cutaneous Malignancies
Advise patients to limit exposure to sunlight and ultraviolet (UV) light, wear protective clothing, and use a sunscreen with a high protection factor. Concomitant phototherapy with UV-B radiation or PUVA‑photochemotherapy is not recommended in patients taking VELSIPITY. If a suspicious skin lesion is observed, patients should immediately report it to their healthcare provider
Posterior Reversible Encephalopathy Syndrome
Advise patients to immediately report to their healthcare provider any symptoms involving sudden onset of severe headache, altered mental status, visual disturbances, or seizure. Inform patients that delayed treatment could lead to permanent neurological consequences
Respiratory Effects
Advise patients that they should contact their healthcare provider if they experience new onset or worsening dyspnea
Immune System Effects after Stopping VELSIPITY
Advise patients that VELSIPITY continues to have effects, such as lowering effects on peripheral lymphocyte count, for up to 5 weeks after the last dose, and to monitor for signs and symptoms of infection during that time
This product’s labeling may have been updated. For the most recent prescribing information, please visit
For Medical Information about VELSIPITY, please visit
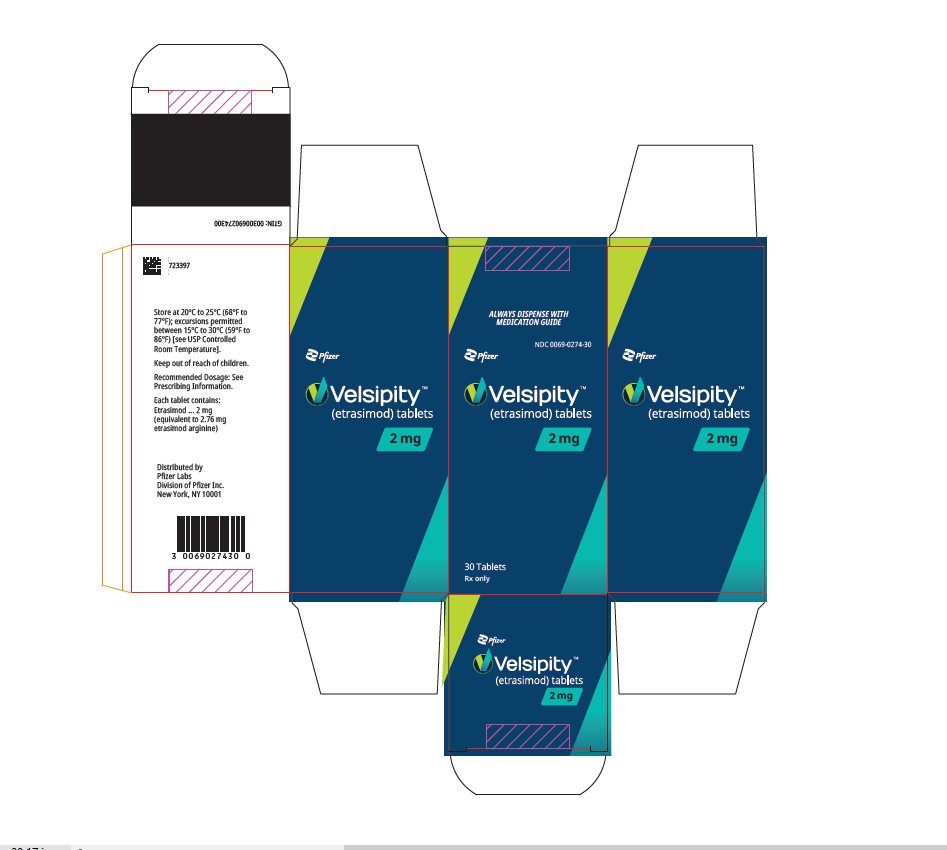
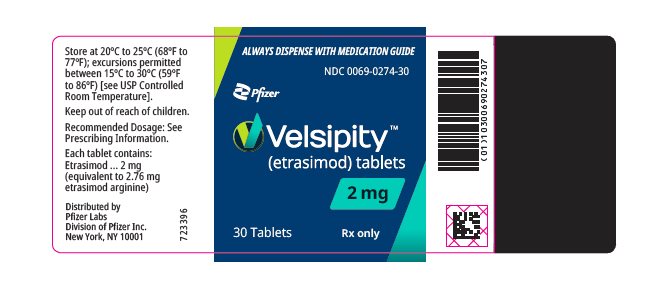
10PRINCIPAL DISPLAY PANEL - 2 mg Tablet Bottle Label
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0069-0274-30
Pfizer
Velsipity™
2 mg
30 Tablets
11PRINCIPAL DISPLAY PANEL - 2 mg Tablet Bottle Carton
ALWAYS DISPENSE WITH
NDC 0069-0274-30
Pfizer
Velsipity™
2 mg
30 Tablets