Brand Name
Ongentys
Generic Name
Opicapone
View Brand Information FDA approval date: April 05, 2024
Classification: Catechol-O-Methyltransferase Inhibitor
Form: Capsule
What is Ongentys (Opicapone)?
ONGENTYS is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease experiencing “off” episodes. ONGENTYS is a catechol-O-methyltransferase inhibitor indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease experiencing “off” episodes.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
ONGENTYS (opicapone)
1INDICATIONS AND USAGE
ONGENTYS is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease (PD) experiencing “off” episodes.
2DOSAGE FORMS AND STRENGTHS
ONGENTYS capsules are available in the following strengths:
- 50 mg capsules with a dark blue opaque cap and dark pink opaque body; axially printed with “OPC” over “50” in white ink, on both the cap and body.
- 25 mg capsules with a light blue opaque cap and light pink opaque body; axially printed with “OPC” over “25” in blue ink, on both the cap and body.
3CONTRAINDICATIONS
ONGENTYS is contraindicated in patients with:
- Concomitant use of non-selective monoamine oxidase (MAO) inhibitors
- Pheochromocytoma, paraganglioma, or other catecholamine secreting neoplasms.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in more detail in other sections of the labeling:
- Cardiovascular Effects with Concomitant Use of Drugs Metabolized by Catechol-O-Methyltransferase (COMT)
- Falling Asleep During Activities of Daily Living and Somnolence
- Hypotension/Syncope
- Dyskinesia
- Hallucinations and Psychosis
- Impulse Control/Compulsive Disorders
- Withdrawal-Emergent Hyperpyrexia and Confusion
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ONGENTYS was evaluated in 265 patients with Parkinson’s disease (PD) in two 14-15 week placebo- and active-controlled (Study 1) or placebo-controlled (Study 2) studies
Adverse Reactions Leading to Discontinuation of Treatment
In Study 1 and Study 2, a total of 8% of ONGENTYS 50 mg-treated patients and 6% of patients who received placebo discontinued due to adverse events. The most common adverse reaction leading to discontinuation was dyskinesia, reported in 3% of ONGENTYS 50 mg-treated patients and 0.4% of patients who received placebo.
Common Adverse Reactions
Adverse reactions that occurred in the pooled studies at an incidence of at least 2% and greater than placebo are presented in Table 1. The most common adverse reactions (incidence at least 4% and greater than placebo) were dyskinesia, constipation, blood creatine kinase increased, hypotension/syncope, and weight decreased.
Table 1:Adverse Reactions with an Incidence of at Least 2% in Patients Treated with ONGENTYS and Greater than on Placebo, in Pooled Study 1 and Study 2
1 Includes hallucinations, hallucinations visual, hallucinations auditory, and hallucinations mixed
2 Includes hypotension, orthostatic hypotension, syncope, and presyncope
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ONGENTYS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish causal relationship to drug exposure.
Injury, poisoning and procedural complications: Fall
Psychiatric disorder: Confusional state
5OVERDOSAGE
No specific antidotes for ONGENTYS are known. As a general measure, removal of ONGENTYS by gastric lavage and/or inactivation by administering activated charcoal should be considered. In managing overdose, provide supportive care, including close medical supervision and monitoring, and consider the possibility of multiple drug involvement. If an over-exposure occurs, call your poison control center at 1-800-222-1222 or www.poison.org.
6DESCRIPTION
ONGENTYS contains opicapone, a peripheral, selective and reversible catechol-O-methyltransferase (COMT) inhibitor. The chemical name of opicapone is 2,5-dichloro-3-(5-(3,4-dihydroxy-5-nitrophenyl)-1,2,4-oxadiazol-3-yl)-4,6-dimethylpyridine-1-oxide with the following structure:
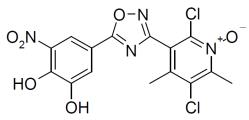
The opicapone molecular formula is C15H10Cl2N4O6; and its molecular weight is 413.17.
Opicapone is a yellow powder/crystalline solid with limited aqueous solubility.
ONGENTYS capsules are intended for oral administration. Each capsule contains 25 mg or 50 mg of opicapone. ONGENTYS also contains the following inactive ingredients: lactose, magnesium stearate, pregelatinized starch, and sodium starch glycolate. The capsule shells contain: FD&C Blue#2, FD&C Red#3, gelatin, and titanium dioxide.
7CLINICAL STUDIES
The efficacy of ONGENTYS for the adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease (PD) experiencing “off” episodes was evaluated in two double-blind, randomized, parallel-group, placebo- and active-controlled (Study 1, NCT01568073), or placebo-controlled (Study 2, NCT01227655) studies of 14-15 week duration. All patients were treated with levodopa/ DOPA decarboxylase inhibitor (DDCI) (alone or in combination with other PD medications). The double-blind period for each study began with a period for levodopa/DDCI dose adjustment (up to 3 weeks), followed by a stable maintenance period of 12 weeks.
Study 1
In Study 1, patients (n=600) were randomized to treatment with one of 3 doses of ONGENTYS. The intention to treat (ITT) population included patients treated with ONGENTYS 50 mg once daily (n=115) or placebo (n=120). Baseline demographic characteristics were similar across all treatment groups: approximately 60% of patients were male, mean age was 64 years, and all patients were Caucasian. Baseline PD characteristics in the treatment groups were: mean duration of PD of 7 years for ONGENTYS 50 mg compared to 7.7 years for placebo, and mean onset of motor fluctuations of 2.2 years prior to study enrollment. Eighty-two percent of patients in both groups used concomitant PD medications in addition to levodopa; the most commonly used were dopamine agonists (68%), amantadine (23%), MAO-B inhibitors (20%), and anticholinergics (5%).
The primary efficacy endpoint was the change in mean absolute OFF-time based on 24-hour patient diaries completed 3 days prior to each of the scheduled visits. ONGENTYS 50 mg significantly reduced mean absolute OFF-time compared to placebo (Table 2).
Table2: Study 1 - Absolute OFF-time (Hours) Change from Baseline to Endpoint
ON-time without troublesome dyskinesia was a secondary efficacy endpoint in Study 1 (Table 3).
Table 3: Study 1 - Absolute ON-time Without Troublesome Dyskinesia (Hours) Change from Baseline to Endpoint
Study 2
In Study 2, patients (n=427) were randomized to treatment with either one of two doses of ONGENTYS once daily (n=283) or placebo (n=144). The intention to treat (ITT) study population included patients treated with ONGENTYS 50 mg once daily (n=147) or placebo (n=135). Baseline demographic characteristics (ONGENTYS 50 mg vs. placebo) were: mean age (66 years vs. 62 years), male (61% vs. 53%), Caucasian (78% vs. 66%) and Asian (21% vs. 31%). Baseline PD characteristics were generally similar across treatment groups with a mean duration of PD of 8.2 years, and a mean onset of motor fluctuations of 3.2 years prior to study enrollment. Eighty-five percent of patients treated with ONGENTYS 50 mg compared to 81% of patients who received placebo used concomitant PD medications in addition to levodopa; the most commonly used were dopamine agonists (70%), amantadine (21%), MAO-B inhibitors (20%), and anticholinergics (12%).
The primary efficacy endpoint was the change in mean absolute OFF-time based on 24-hour patient diaries completed 3 days prior to each of the scheduled visits. ONGENTYS 50 mg significantly reduced mean absolute OFF-time compared to placebo (Table 4).
Table 4: Study 2 - Absolute OFF-time (Hours) Change from Baseline to Endpoint
ON-time without troublesome dyskinesia was a secondary efficacy endpoint in Study 2 (Table 5).
Table 5: Study 2 - Absolute ON-time Without Troublesome Dyskinesia (Hours) Change from Baseline to Endpoint
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
Administration
Instruct patients and/or caregivers that ONGENTYS capsules should be taken at bedtime. Inform patients to not eat food for 1 hour before and for at least 1 hour after intake of ONGENTYS
Concomitant Medications
Certain medications can cause an interaction with ONGENTYS. Advise patients and/or caregivers to inform their healthcare provider of all the medicines the patient is taking, including over-the-counter medicines, dietary supplements, and herbal products
Falling Asleep During Activities of Daily Living
Advise patients and/or caregivers that somnolence has been reported with ONGENTYS. Patients treated with dopaminergic medications have reported falling asleep while engaged in activities of daily living. These adverse reactions may affect some patients’ ability to drive and operate machinery safely
Hypotension/Syncope
Advise patients that ONGENTYS may cause hypotension or syncope
Dyskinesia
Advise patients that ONGENTYS may cause dyskinesia or exacerbate pre-existing dyskinesia
Hallucinations and Psychosis
Advise patients that ONGENTYS may cause hallucinations, delusions, or aggressive behavior and they should report any of these adverse reactions to their healthcare provider
Impulse Control/Compulsive Disorders
Inform patients of the potential for experiencing intense urges to gamble, increased sexual urges, intense urges to spend money, binge eating, and other intense urges and the inability to control these urges while taking ONGENTYS and one or more medications that increase central dopaminergic tone that are generally used for the treatment of PD. Advise patients that they should report any of these adverse reactions to their healthcare provider
Withdrawal-Emergent Hyperpyrexia and Confusion
Advise patients to contact their healthcare provider before stopping ONGENTYS. Tell patients to inform their healthcare provider if they develop symptoms such as fever, confusion, or severe muscle stiffness after stopping ONGENTYS
For further information on ONGENTYS, call 1-877-835-5472 or visit www.ongentys.com
Distributed by:
Rev. 10-2025-02
Under license from BIAL-Portela & C
ONGENTYS is a registered trademark of BIAL-Portela & C
9PATIENT INFORMATION
This Patient Information has been approved by the U.S. Food and Drug Administration Rev. 12-2023-00
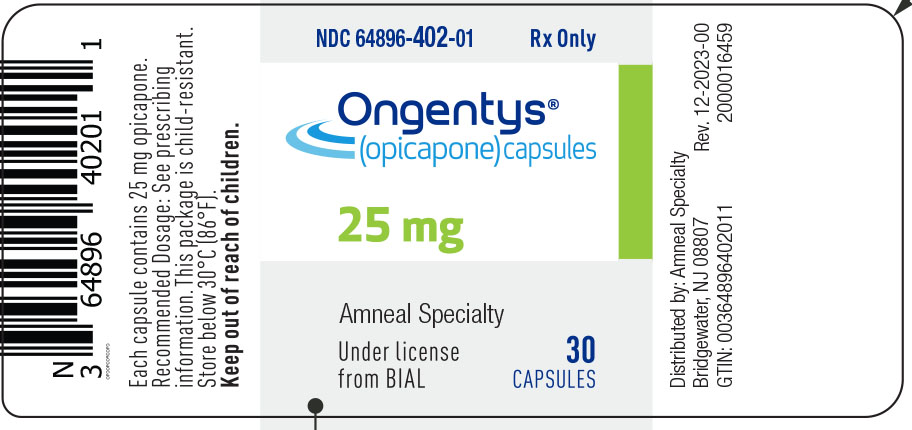
10PRINCIPAL DISPLAY PANEL
NDC 64896-402-01
Ongentys
(opicapone) capsules
25 mg
30 Capsules
Rx only
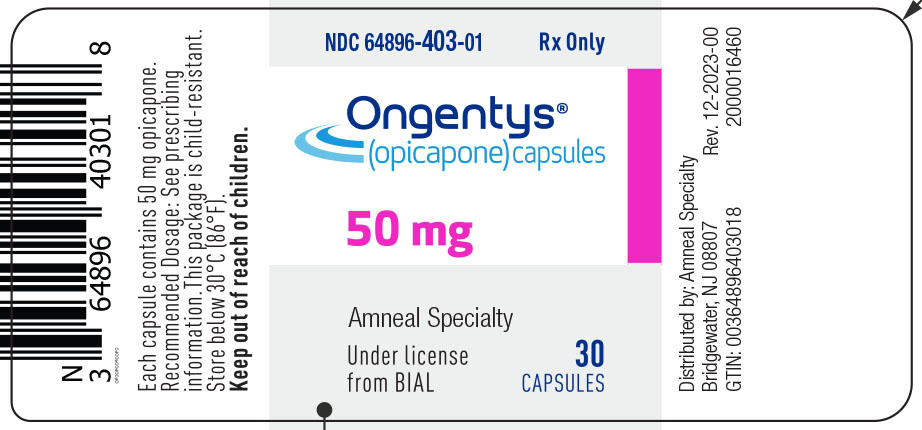
11PRINCIPAL DISPLAY PANEL
NDC 64896-403-01
Ongentys
(opicapone) capsules
50 mg
30 Capsules
Rx only