Generic Name
Riluzole
Brand Names
Tiglutik, Teglutik
FDA approval date: June 18, 2013
Classification: Benzothiazole
Form: Tablet, Liquid
What is Tiglutik (Riluzole)?
Riluzole is indicated for the treatment of amyotrophic lateral sclerosis . Riluzole is indicated for the treatment of amyotrophic lateral sclerosis
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
TIGLUTIK (Riluzole)
1INDICATIONS AND USAGE
TIGLUTIK is indicated for the treatment of amyotrophic lateral sclerosis (ALS).
2DOSAGE FORMS AND STRENGTHS
Oral suspension: 50 mg/10 mL (5 mg/mL) slightly brown, opaque, homogeneous suspension in a 300-mL multiple-dose amber bottle.
3CONTRAINDICATIONS
TIGLUTIK is contraindicated in patients with a history of severe hypersensitivity reactions to riluzole or to any of its components (anaphylaxis has occurred)
4ADVERSE REACTIONS
The following adverse reactions are described below and elsewhere in the labeling:
- Hepatic Injury
- Neutropenia
- Interstitial Lung Disease
- Pancreatitis
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of riluzole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Acute hepatitis and icteric toxic hepatitis
- Renal tubular impairment
- Pancreatitis
5OVERDOSAGE
Reported symptoms of overdose following ingestion of riluzole ranging from 1.5 to 3 grams (30 to 60 times the recommended dose) included acute toxic encephalopathy, coma, drowsiness, memory loss, and methemoglobinemia.
No specific antidote for the treatment of TIGLUTIK overdose is available. For current information on the management of poisoning or overdosage, contact a certified poison control center.
6DESCRIPTION
Riluzole is a member of the benzothiazole class. The chemical designation for riluzole is 2-amino-6-(trifluoromethoxy)benzothiazole. Its molecular formula is C
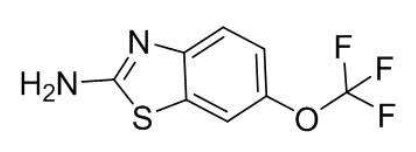
Riluzole is a white to slightly yellow powder that is very soluble in dimethylformamide, dimethylsulfoxide, and methanol; freely soluble in dichloromethane; sparingly soluble in 0.1 N HCl; and very slightly soluble in water and in 0.1 N NaOH.
TIGLUTIK (50 mg/10mL) oral suspension is a slightly brown, opaque, homogeneous suspension containing 50 mg of riluzole per 10 mL of suspension.
TIGLUTIK also contains the following inactive ingredients: magnesium aluminum silicate, noncrystallizing sorbitol solution, polyoxyl 20 cetostearyl ether, purified water, saccharin sodium, simethicone emulsion, sodium lauryl sulfate, and xanthan gum.
7CLINICAL STUDIES
The efficacy of TIGLUTIK is based upon bioavailability studies comparing oral riluzole tablets to TIGLUTIK oral suspension
The efficacy of riluzole was demonstrated in two studies (Study 1 and 2) that evaluated 50 mg riluzole oral tablets twice daily in patients with amyotrophic lateral sclerosis (ALS). Both studies included patients with either familial or sporadic ALS, disease duration of less than 5 years, and baseline forced vital capacity greater than or equal to 60% of normal.
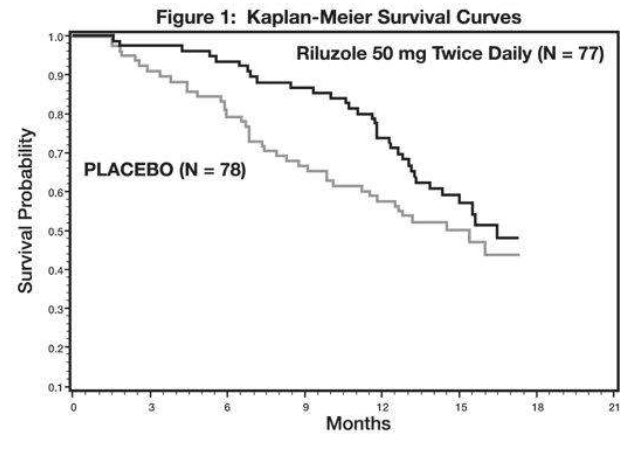
Study 1 was a randomized, double-blind, placebo-controlled clinical study that enrolled 155 patients with ALS. Patients were randomized to receive riluzole 50 mg twice daily (n=77) or placebo (n=78) and were followed for at least 13 months (up to a maximum duration of 18 months). The clinical outcome measure was time to tracheostomy or death.
The time to tracheostomy or death was longer for patients receiving riluzole compared to placebo. There was an early increase in survival in patients receiving riluzole compared to placebo. Figure 1 displays the survival curves for time to death or tracheostomy. The vertical axis represents the proportion of individuals alive without tracheostomy at various times following treatment initiation (horizontal axis). Although these survival curves were not statistically significantly different when evaluated by the analysis specified in the study protocol (Logrank test p=0.12), the difference was found to be significant by another appropriate analysis (Wilcoxon test p=0.05). As seen in Figure 1, the study showed an early increase in survival in patients given riluzole. Among the patients in whom the endpoint of tracheostomy or death was reached during the study, the difference in median survival between the riluzole 50 mg twice daily and placebo groups was approximately 90 days.
Figure 1. Time to Tracheostomy or Death in ALS Patients in Study 1 (Kaplan-Meier Curves)
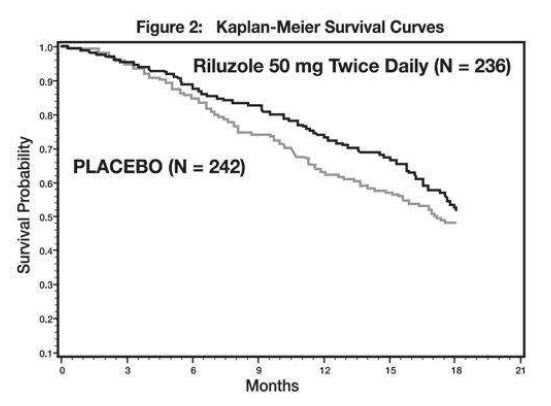
Study 2 was a randomized, double-blind, placebo-controlled clinical study that enrolled 959 patients with ALS. Patients were randomized to riluzole 50 mg twice daily (n=236) or placebo (n=242) and were followed for at least 12 months (up to a maximum duration of 18 months). The clinical outcome measure was time to tracheostomy or death.
The time to tracheostomy or death was longer for patients receiving riluzole compared to placebo. Figure 2 displays the survival curves for time to death or tracheostomy for patients randomized to either riluzole 100 mg per day or placebo. Although these survival curves were not statistically significantly different when evaluated by the analysis specified in the study protocol (Logrank test p=0.076), the difference was found to be significant by another appropriate analysis (Wilcoxon test p=0.05). Not displayed in Figure 2 are the results of riluzole 50 mg per day (one-half of the recommended daily dose), which could not be statistically distinguished from placebo, or the results of riluzole 200 mg per day (two times the recommended daily dose), which were not distinguishable from the 100 mg per day results. Among the patients in whom the endpoint of tracheostomy or death was reached during the study, the difference in median survival between riluzole and placebo was approximately 60 days.
Although riluzole improved survival in both studies, measures of muscle strength and neurological function did not show a benefit.
Figure 2. Time to Tracheostomy or Death in ALS Patients in Study 2 (Kaplan-Meier Curves)
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
9PRINCIPAL DISPLAY PANEL - 300 mL Bottle Carton
NDC 70726-0303-1
R
Tiglutik
For Oral Administration
Contents of this package:
- Two bottles, each containing 300 mL
- Two 10 mL oral syringes
- Two syringe bottle adapters
- Two syringe tip caps
- One Prescribing Information, including
This product is a liquid suspension and is
600 mL (two bottles/300 mL each)

10PRINCIPAL DISPLAY PANEL - 300 mL Bottle
NDC 70726-0303-2
R
Tiglutik
riluzole
oral suspension
50 mg/10 mL (5 mg/mL)
riluzole
oral suspension
50 mg/10 mL (5 mg/mL)
For Oral Administration
Shake gently before use
Shake gently before use
300 mL
Discard unused portion 15 days after first opening.
ITF Pharma
Manufactured for:
