Brand Name
Oxistat
Generic Name
Oxiconazole
View Brand Information FDA approval date: March 07, 2016
Classification: Azole Antifungal
Form: Lotion, Cream
What is Oxistat (Oxiconazole)?
Oxiconazole nitrate cream is indicated for the topical treatment of the following dermal infections: tinea pedis, tinea cruris, and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum. Oxiconazole nitrate cream is indicated for the topical treatment of tinea versicolor due to Malassezia furfur. Oxiconazole nitrate cream may be used in pediatric patients for tinea corporis, tinea cruris, tinea pedis, and tinea versicolor; however, these indications for which oxiconazole nitrate cream has been shown to be effective rarely occur in children below the age of 12.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Oxistat (oxiconazole nitrate)
1DESCRIPTION
OXISTAT
Chemically, oxiconazole nitrate is 2',4'-dichloro-2-imidazol-1-ylacetophenone (
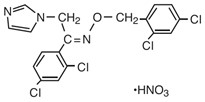
Oxiconazole nitrate is a nearly white crystalline powder, soluble in methanol; sparingly soluble in ethanol, chloroform, and acetone; and very slightly soluble in water.
OXISTAT Lotion contains 10 mg of oxiconazole per gram of lotion in a white to off-white, opaque lotion base of purified water, white petrolatum, stearyl alcohol, propylene glycol, polysorbate 60, cetyl alcohol, and benzoic acid 0.2% as a preservative.
2INDICATIONS AND USAGE
OXISTAT Lotion is indicated for the topical treatment of the following dermal infections: tinea pedis, tinea cruris, and tinea corporis due to
3CONTRAINDICATIONS
OXISTAT Lotion is contraindicated in individuals who have shown hypersensitivity to any of their components.
4WARNINGS
OXISTAT (oxiconazole nitrate) Lotion, 1% is for topical use only and not for oral, ophthalmic or intravaginal use.
5ADVERSE REACTIONS
In a controlled, multicenter clinical trial of 269 patients treated with oxiconazole nitrate
The following additional adverse experiences have been reported with the topical use of oxiconazole nitrate: irritation and allergic contact dermatitis, folliculitis, erythema, papules, fissure, maceration, rash, and nodules.
To report SUSPECTED ADVERSE REACTIONS, contact ANI Pharmaceuticals, Inc. at 1-800-308-6755 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
6OVERDOSAGE
When 5% oxiconazole cream (5 times the concentration of the marketed product) was applied at a rate of 1 g/kg to approximately 10% of body surface area of a group of 40 male and female rats for 35 days, 3 deaths and severe dermal inflammation were reported. No overdoses in humans have been reported with use of oxiconazole nitrate cream or lotion.
7DOSAGE AND ADMINISTRATION
OXISTAT Lotion should be applied to affected and immediately surrounding areas once to twice daily in patients with tinea pedis, tinea corporis, or tinea cruris. Tinea corporis and tinea cruris should be treated for 2 weeks and tinea pedis for 1 month to reduce the possibility of recurrence. If a patient shows no clinical improvement after the treatment period, the diagnosis should be reviewed.
8CLINICAL STUDIES
The following definitions were applied to the clinical and microbiological outcomes in patients enrolled in the clinical trial that form the basis for the approval of OXISTAT Lotion.
8.1Definitions
- Mycological Cure: No evidence (culture and KOH preparation) of the baseline (original) pathogen in a specimen from the affected area taken at the 2-week post-treatment visit (for tinea [pityriasis] versicolor, mycological cure was limited to KOH only).
- Treatment Success:
8.2Tinea Pedis
The clinical trial for the lotion formulation line extension involved 332 evaluable patients with clinically and microbiologically established tinea pedis. Of these evaluable patients, 64% were diagnosed with hyperkeratotic plantar tinea pedis and 28% with interdigital tinea pedis. Seventy-seven percent (77%) had disease secondary to infection with
The results of this clinical trial at the 2-week post-treatment follow-up visit are shown in the following table:
In this study, the improvement and cure rates of the b.i.d.- and q.d.-treated groups did not differ significantly (95% confidence interval) from each other but were statistically (95% confidence interval) superior to the vehicle-treated group.
9HOW SUPPLIED
OXISTAT
Store at 20° to 25°C (68° to 77°F); excursions permitted at 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Shake well before using.
OXISTAT is a registered trademark of Fougera Pharmaceuticals, Inc. and is licensed to ANI Pharmaceuticals, Inc.
Distributed by:
N7000 Rev 05/22
10PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 62559-