Brand Name
Qelbree
Generic Name
Viloxazine
View Brand Information FDA approval date: April 02, 2021
Classification: Norepinephrine Reuptake Inhibitor
Form: Capsule
What is Qelbree (Viloxazine)?
Qelbree is indicated for the treatment of Attention-Deficit Hyperactivity Disorder in pediatric patients 6 to 17 years of age. Qelbree is a selective norepinephrine reuptake inhibitor indicated for the treatment of Attention Deficit Hyperactivity Disorder in pediatric patients 6 to 17 years of age
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Phase 4, Randomized, Double-Blind, Multicenter, Placebo-Controlled, Parallel-Group Study of the Efficacy and Safety of SPN-812 in Preschool-Age Children (4 to 5 Years Old) With Attention-Deficit/Hyperactivity Disorder (ADHD)
Summary: This study will evaluate the efficacy and safety of SPN-812 (viloxazine extended release) in children 4 to 5 years of age with ADHD.
Related Latest Advances
Brand Information
QELBREE (viloxazine hydrochloride)
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
In clinical studies, higher rates of suicidal thoughts and behavior were reported in patients with ADHD treated with Qelbree than in patients treated with placebo. Closely monitor all Qelbree-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors
1INDICATIONS AND USAGE
Qelbree is indicated for the treatment of Attention-Deficit Hyperactivity Disorder (ADHD) in adults and pediatric patients 6 years and older.
2DOSAGE FORMS AND STRENGTHS
Qelbree (viloxazine extended-release capsules) are available as:
100 mg: yellow opaque body and cap (printed "SPN" on the cap, "100" on the body)
150 mg: lavender opaque body and cap (printed "SPN" on the cap, "150" on the body)
200 mg: light green opaque body and cap (printed "SPN" on the cap, "200" on the body)
3CONTRAINDICATIONS
Qelbree is contraindicated in patients:
- receiving concomitant treatment with monoamine oxidase inhibitors (MAOI), or within 14 days following discontinuing an MAOI, because of an increased risk of hypertensive crisis
- receiving concomitant administration of sensitive CYP1A2 substrates or CYP1A2 substrates with a narrow therapeutic range
4ADVERSE REACTIONS
The following serious adverse reactions are described in other sections of the labeling:
- Suicidal Thoughts and Behaviors
- Blood Pressure and Heart Rate Increases
- Activation of Mania or Hypomania
- Somnolence and Fatigue
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of Qelbree has been evaluated in 1118 pediatric patients (6 to 17 years of age) with ADHD exposed to one or more doses in short-term (6 to 8 week), randomized, double-blind, placebo-controlled trials. A total of 682 pediatric patients 6 to 17 years of age were treated for at least 6 months, and 347 pediatric patients 6 to 17 years of age for at least 12 months with Qelbree.
The safety of Qelbree has been evaluated in 189 adult patients (18 to 60 years of age) with ADHD exposed to one or more doses in a short-term (6 week), randomized, double-blind, placebo-controlled trial. A total of 277 adult patients with ADHD have been exposed to one or more doses of Qelbree. Eighty-four adult patients were treated for at least 6 months, and 22 adult patients for at least 12 months.
5DESCRIPTION
Qelbree contains viloxazine, a selective norepinephrine reuptake inhibitor, in the form of viloxazine hydrochloride which is (±)-2-[(2-ethoxyphenoxy)methyl]morpholine hydrochloride. The molecular formula is C
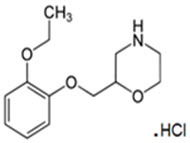
Viloxazine hydrochloride is a white to off-white powder. Viloxazine hydrochloride is soluble in water, 0.1N HCl and aqueous solutions of pH 9.5 and lower. Viloxazine hydrochloride is sparingly soluble in methanol, very slightly soluble in acetonitrile, acetic acid and isopropyl alcohol, and practically insoluble in ethyl acetate.
Qelbree extended-release capsules are intended for oral administration. Each extended-release capsule contains 100 mg, 150 mg, and 200 mg of viloxazine free base equivalent to 115mg, 173mg, and 231mg, respectively, of viloxazine hydrochloride salt.
The inactive ingredients are: Ammonium hydroxide, black iron oxide, butyl alcohol, corn starch, ethylcellulose, FD&C Blue #1, FD&C Red #28, FD&C Yellow #5, FD&C Yellow #6, FD&C Yellow #10, gelatin, hypromellose, isopropyl alcohol, lactose monohydrate, medium chain triglycerides, oleic acid, polyethylene glycol, potassium hydroxide, propylene glycol, shellac, strong ammonia solution, sucrose, talc, triacetin, titanium dioxide.
6PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
7PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle Label
30 Capsules
Qelbree
100 mg
Once daily
ATTENTION PHARMACIST:
Rx only

8PRINCIPAL DISPLAY PANEL - 150 mg Capsule Bottle Label
30 Capsules
Qelbree
150 mg
Once daily
ATTENTION PHARMACIST:
Rx only

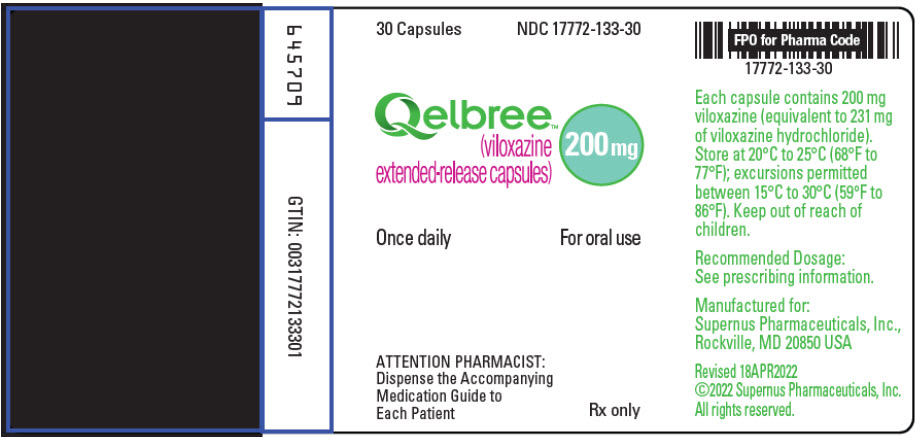
9PRINCIPAL DISPLAY PANEL - 200 mg Capsule Bottle Label
30 Capsules
Qelbree
200 mg
Once daily
ATTENTION PHARMACIST:
Rx only