Brand Name
Nateglinide
View Brand InformationFDA approval date: September 09, 2009
Classification: Glinide
Form: Tablet
What is Nateglinide?
Nateglinide tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Limitations of Use: Nateglinide should not be used in patients with type 1 diabetes mellitus or for the treatment of diabetic ketoacidosis. Nateglinide is a glinide indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Limitations of use: Not for treating type 1 diabetes mellitus or diabetes ketoacidosis
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
NATEGLINIDE (NATEGLINIDE)
1INDICATIONS AND USAGE
Nateglinide tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
2DOSAGE AND ADMINISTRATION
The recommended dose of nateglinide tablets is 120 mg orally three times daily before meals.
The recommended dose of nateglinide tablets is 60 mg orally three times daily before meals in patients who are near glycemic goal when treatment is initiated.
Instruct patients to take nateglinide tablets 1 to 30 minutes before meals.
In patients who skip meals, instruct patients to skip the scheduled dose of nateglinide tablets to reduce the risk of hypoglycemia {see
3DOSAGE FORMS AND STRENGTHS
- 60 mg tablets: Pink, round, beveled edge tablet with "C" debossed on one side and "123" on the other side
- 120 mg tablets: Yellow, ovaloid tablet with "C" debossed on one side and "125" on the other side
4CONTRAINDICATIONS
Nateglinide is contraindicated in patients with a history of hypersensitivity to nateglinide or its inactive ingredients.
5ADVERSE REACTIONS
The following serious adverse reaction is also described elsewhere in the labeling:
Hypoglycemia [see
5.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials, approximately 2,600 patients with type 2 diabetes mellitus were treated with nateglinide. Of these, approximately 1,335 patients were treated for 6 months or longer and approximately 190 patients for one year or longer. Table 1 shows the most common adverse reactions associated with nateglinide.
5.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of nateglinide tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hypersensitivity reactions: Rash, itching, and urticaria
- Hepatobiliary Disorders: Jaundice, cholestatic hepatitis, and elevated liver enzymes
6DRUG INTERACTIONS
Table 2 includes a list of drugs with clinically important drug interactions when concomitantly administered or withdrawn with nateglinide tablets and instructions for managing or preventing them.
7OVERDOSAGE
There have been no instances of overdose with nateglinide in clinical trials. However, an overdose may result in an exaggerated glucose-lowering effect with the development of hypoglycemic symptoms. Hypoglycemic symptoms without loss of consciousness or neurological findings should be treated with oral glucose and adjustments in dosage and/or meal patterns. Severe hypoglycemic reactions with coma, seizure, or other neurological symptoms should be treated with intravenous glucose. As nateglinide are highly protein bound, dialysis is not an efficient means of removing it from the blood.
8DESCRIPTION
Nateglinide, USP is an oral blood glucose-lowering drug of the glinide class. Nateglinide, USP (-)-N-[(trans-4-isopropylcyclohexane)carbonyl]-D-phenylalanine, is structurally unrelated to the oral sulfonylurea insulin secretagogues.
The structural formula is as shown:
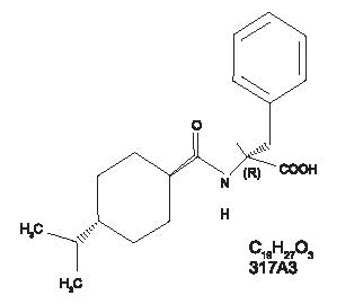
Nateglinide is a white or almost white powder with a molecular weight of 317.42. It is freely soluble in methanol, methylene chloride and in alcohol, soluble in ether, sparingly soluble in acetonitrile and in octanol, practically insoluble in water. Nateglinide tablets contain 60 mg, or 120mg, of nateglinide for oral administration.
Inactive ingredients: colloidal silicon dioxide, corn starch, croscarmellose sodium, hypromellose, mannitol, iron oxide (yellow and red), polyethylene glycol, povidone, pre-gelatinized starch, sodium lauryl sulphate, sodium starch glycolate, sodium stearyl fumarate, talc and titanium dioxide.
Film-coating material contains opadry pink and opadry yellow for the 60 mg and 120 mg. Opadry pink contains hypromellose, iron oxide red, macrogol and titanium dioxide. Opadry yellow contains hypromellose, iron oxides (yellow and red), macrogol, titanium dioxide, and talc.
9PRINCIPAL DISPLAY PANEL - 60 mg Tablet Bottle Label
NDC 75834-205-01
NATEGLINIDE
60 mg
100 Tablets
NIVAGEN
Rx only
10PRINCIPAL DISPLAY PANEL - 120 mg Tablet Bottle Label
NDC 75834-206-01
NATEGLINIDE
120 mg
100 Tablets
NIVAGEN
Rx only


