Brand Name
DDAVP
Generic Name
Desmopressin Acetate
View Brand Information FDA approval date: May 01, 1984
Classification: Factor VIII Activator
Form: Injection, Spray, Tablet, Solution
What is DDAVP (Desmopressin Acetate)?
Central Diabetes Insipidus Desmopressin acetate tablets are indicated as antidiuretic replacement therapy in the management of central diabetes insipidus and for the management of the temporary polyuria and polydipsia following head trauma or surgery in the pituitary region. Desmopressin acetate is ineffective for the treatment of nephrogenic diabetes insipidus. Patients were selected for therapy based on the diagnosis by means of the water deprivation test, the hypertonic saline infusion test, and/or response to antidiuretic hormone. Continued response to desmopressin acetate can be monitored by measuring urine volume and osmolality. Primary Nocturnal Enuresis Desmopressin acetate tablets are indicated for the management of primary nocturnal enuresis. Desmopressin acetate may be used alone or as an adjunct to behavioral conditioning or other non-pharmacologic intervention.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
DDAVP (Desmopressin Acetate)
WARNING: HYPONATREMIA
DDAVP can cause hyponatremia. Severe hyponatremia can be life-threatening, leading to seizures, coma, respiratory arrest, or death
DDAVP is contraindicated in patients at increased risk of severe hyponatremia, such as patients with excessive fluid intake, illnesses that can cause fluid or electrolyte imbalances, and in those using loop diuretics or systemic or inhaled glucocorticoids
Ensure the serum sodium concentration is normal before starting or resuming DDAVP. Measure serum sodium within 7 days and approximately 1 month after initiating therapy, and periodically during treatment. More frequently monitor serum sodium in patients 65 years of age and older and in patients at increased risk of hyponatremia
If hyponatremia occurs, DDAVP may need to be temporarily or permanently discontinued
1DOSAGE FORMS AND STRENGTHS
Injection: DDAVP is a sterile, aqueous, colorless solution available as:
- 4 mcg/mL in single-dose ampule
- 40 mcg/10 mL (4 mcg/mL) in multiple-dose vial
2CONTRAINDICATIONS
DDAVP Injection is contraindicated in patients with known hypersensitivity to desmopressin acetate or to any of the components of DDAVP Injection
DDAVP Injection is contraindicated in patients with the following conditions due to an increased risk of hyponatremia:
- Moderate to severe renal impairment defined as a creatinine clearance below 50 mL/min
- Hyponatremia or a history of hyponatremia
- Known or suspected syndrome of inappropriate antidiuretic hormone (SIADH) secretion
- Polydipsia
- Concomitant use with loop diuretics
- Concomitant use with systemic or inhaled glucocorticoids
- During illnesses that can cause fluid or electrolyte imbalance, such as gastroenteritis, salt-wasting nephropathies, or systemic infection
DDAVP Injection is contraindicated in patients with the following conditions because fluid retention increases the risk of worsening the underlying condition:
- Heart failure
- Uncontrolled hypertension
3ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hyponatremia
- Hypotension and Hypertension
- Increased risk of thrombosis in patients with von Willebrand's Disease Type IIB
- Hypersensitivity reactions
- Fluid retention
The following adverse reactions have been identified during post-approval use of DDAVP Injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular: Hypertension, hypotension, tachycardia, thrombotic events, fluid retention
4OVERDOSAGE
Overdosage of DDAVP Injection leads to prolonged duration of action with an increased risk of water retention and hyponatremia. Signs of overdose may include headaches, abdominal cramps, nausea, facial flushing, confusion, drowsiness, problems with passing urine and rapid weight gain due to fluid retention
There is no known specific antidote for desmopressin acetate or DDAVP Injection 4 mcg/mL.
5DESCRIPTION
DDAVP Injection (desmopressin acetate) is a synthetic vasopressin analog for intravenous or subcutaneous use. It is chemically defined as follows:
Mol. Wt. 1183.34
Empirical Formula: C
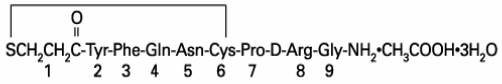
1-(3-mercaptopropionic acid)-8-D-arginine vasopressin monoacetate (salt) trihydrate.
DDAVP solution for injection is a sterile solution in a 1 mL single-dose ampule: Each mL contains 4 mcg of desmopressin acetate which is equivalent to 3.6 mcg of desmopressin free base. The inactive ingredients are sodium chloride, hydrochloric acid and water for injection. Sodium chloride is used to adjust tonicity. Hydrochloric acid is used to adjust pH.
DDAVP solution for injection is a sterile solution in a 10 mL multiple-dose vial: Each mL contains 4 mcg of desmopressin acetate which is equivalent to 3.6 mcg of desmopressin free base. The inactive ingredients are sodium chloride, chlorobutanol (5 mg per mL), hydrochloric acid and water for injection. Sodium chloride is used to adjust tonicity. Hydrochloric acid is used to adjust pH.
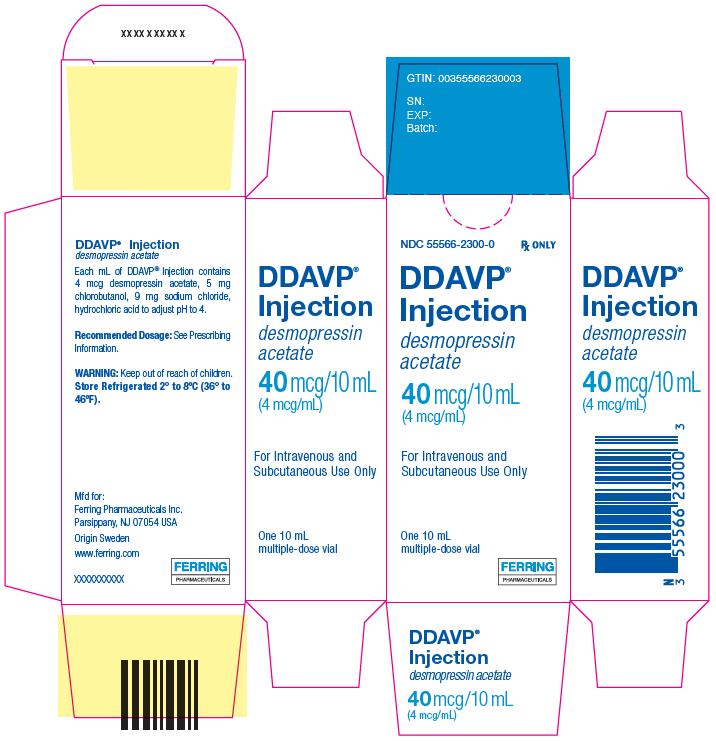
6PRINCIPAL DISPLAY PANEL - 10 mL Vial Carton
NDC 55566-2300-0
Rx ONLY
DDAVP
desmopressin
40 mcg/10 mL
For Intravenous and
One 10 mL
FERRING
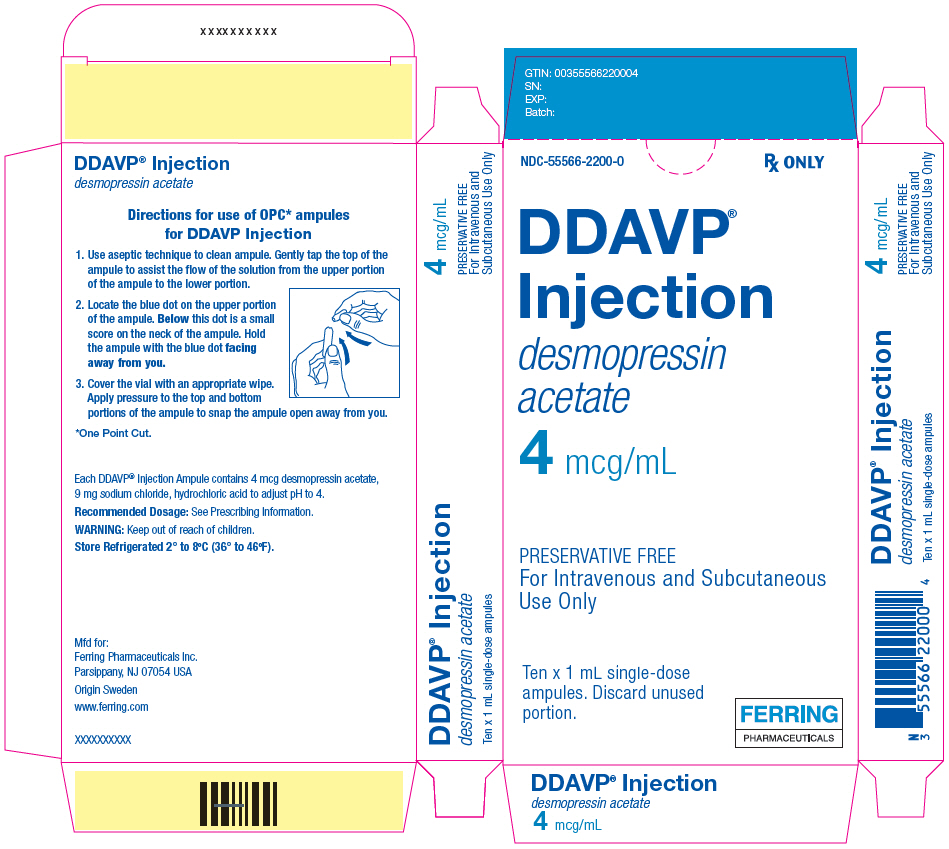
7PRINCIPAL DISPLAY PANEL - 1 mL Ampule Carton
NDC-55566-2200-0
Rx ONLY
DDAVP
desmopressin
4 mcg/mL
PRESERVATIVE FREE
Ten x 1 mL single-dose
FERRING